1. INTRODUCTION
Alloy 625 (UNS N06625) is a nickel based alloy. It is used extensively in the oil and gas and petrochemical industries when a material with high strength and good corrosion resistance is required, but process conditions preclude the use of duplex/super duplex stainless steel. Its microstructure and properties are dependent on its precise chemical composition and processing history.
Alloy 625 is normally considered to be a solid solution alloy. It gets its strength mainly from the high levels of chromium and molybdenum with the niobium and, to a lesser extent, iron content providing additional solid solution strengthening [1].
Alloy 625 also contains additions of aluminium and titanium. These are present essentially for refining purposes and are generally kept low in comparison to other alloys, such as Alloy 718, to improve weldability. However, if sufficient niobium, aluminium and titanium are present, precipitation hardening is possible [1].
It is the high levels of chromium and molybdenum that give Alloy 625 its excellent corrosion resistance.
This is Part 1 of an article covering the invention and development of Alloy 625 and is based on a paper produced by H. L. Eiselstein and D. J. Tillack [2]; the inventors of Alloy 625. The effects of the different alloying elements on strength and creep resistance are discussed as well as the influence of heat treatment on the properties of the finished product. This article touches on the factors influencing the corrosion resistance and metallurgy of Alloy 625, but these will be covered in greater detail in a later article.
2. INVENTION
Alloy 625 was invented in the 1950s by Inco Alloys International, Inc. [2]. It was originally intended to be used as a replacement for Type 316 stainless steel in super critical steam power plants. Apart from strength, one of the key features of a main steam-line material is metallurgical stability, i.e. the material will not age (change during service).
Initial work concentrated on evaluating the strengthening effects of the major alloying elements, i.e. chromium, molybdenum, niobium, aluminium and titanium. It was found that the strengthening effect of each element in isolation was limited, so it was decided to use molybdenum and niobium in combination with varying amounts of nickel. Because a key requirement of the material was its metallurgical stability, tensile samples of each alloy combination were given a simple ageing treatment (e.g. 704°C for 16 hours) and tested at room temperature. It was hoped that these tests would indicate any tendencies for age-hardening or embrittlement. They did in spectacular fashion as can be seen from Figure 1 [3].
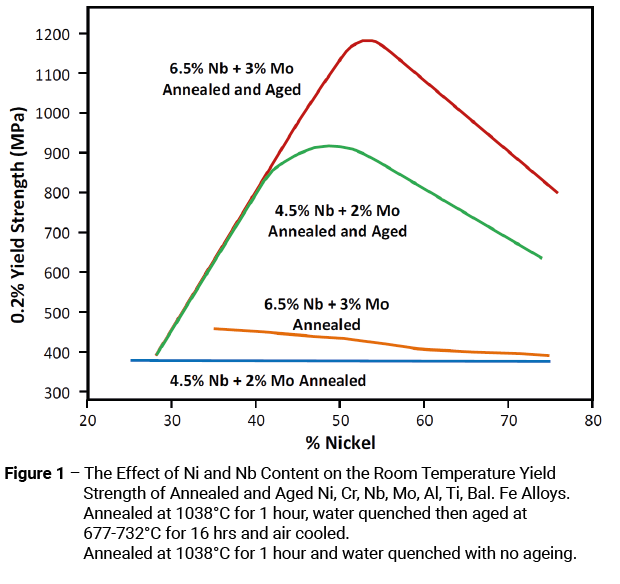
The net result of these tests was to delay the development of Alloy 625 as attention shifted to the development of a different alloy, namely Alloy 718.
By the time development of Alloy 625 resumed, the market for a nickel-based alloy for super critical steam no longer existed. The composition of the alloy under evaluation at that time was approximately 60% Ni, 15% Cr, 3% Nb, 2% Mo, 0.5% AI and 0.5% Ti with Fe making up the balance. In the annealed condition, this alloy was only a little stronger than Alloy 600 (UNS N06600); as such it offered little advantage over existing alloys. To make it more attractive, it was felt that the alloy needed higher room temperature tensile properties.
To achieve this, the chromium and molybdenum contents were raised to their current levels of 22% and 9%, respectively. Fortunately, the use of these elements not only increased the alloy’s room temperature tensile properties but also greatly increased its corrosion resistance. It was the combination of high strength and good corrosion resistance that altered the course of development of the alloy and increased its marketing opportunities.
After several years of discovering how various elements affected the properties of the alloy system, a patent application was submitted on January 24, 1962. Patent № 3,160,500 was issued to H. L. Eiselstein and J. Gadbut on December 8, 1964. The composition has changed slightly since the patent was issued. The present composition for Alloy 625 is shown in Table 1.

Table 1 – Alloy 625 (UNS N06625) Composition (%)
3. EFFECTS OF ELEMENTS ON STRENGTH
Even with the disappearance of the super critical steam market, the development of Alloy 625 was still concentrated on its use at high temperature. Figure 2 shows the effect of nickel on the 649°C stress-rupture strength of annealed Alloy 625 and indicates a peak at around 57%. Figure 3 shows the effect of niobium on yield strength. It can be seen that in the annealed condition niobium has very little effect on the yield strength. This is also the case in the annealed and aged condition at niobium levels below 2%. However, once the niobium level exceeds about 3%, the effect becomes significant; this would appear to coincide with the observation that the solubility of niobium in the alloy was found to be around 2.5%.
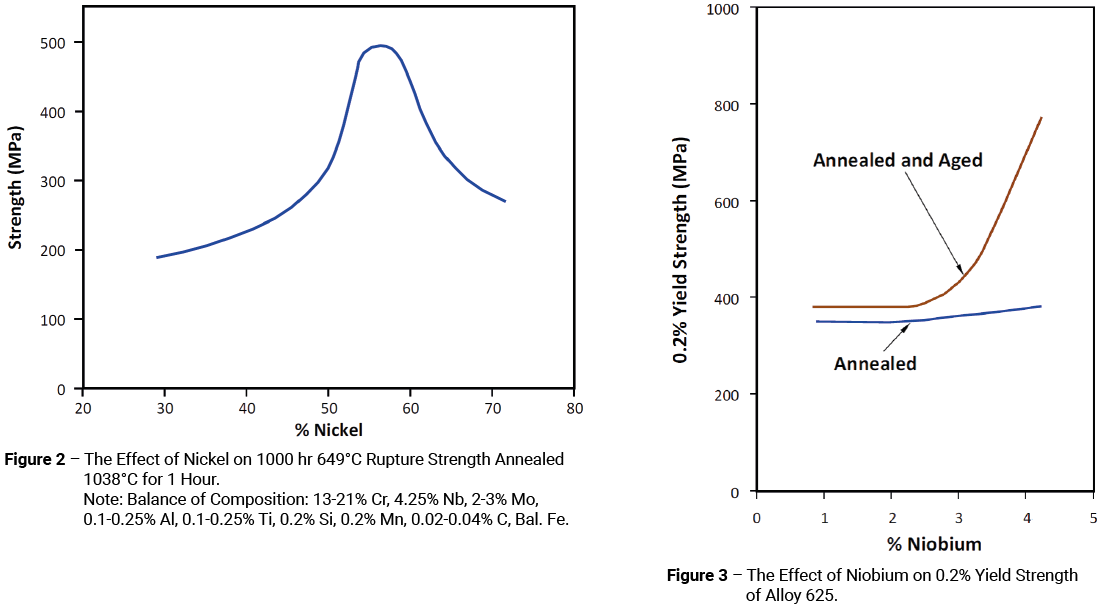
It was already known that molybdenum increased the solid solution strengthening of the alloy. What wasn’t known was that molybdenum also increased its age-hardening response. Whether the increase in age-hardening response was due solely to the effect of the molybdenum or to some interaction with niobium was never investigated; although, it is interesting to note that the solubility of niobium in the alloy decreased with increasing Cr + Mo content, which may have some relevance in this respect.
As expected, increasing the chromium content from 16% to 22% increased the strength of the solid solution, but appeared to have no effect on the age-hardening response of the alloy.
Molybdenum, chromium and especially niobium impart creep strength to the alloy. These effects are shown in Figures 4 and 5.
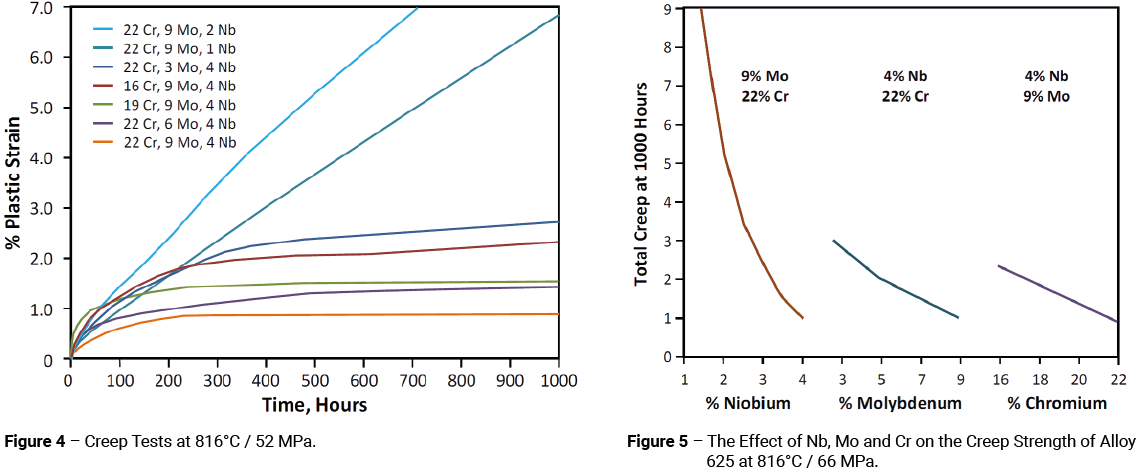
Aluminium and titanium were intentionally kept low to minimise the age-hardenability of the alloy. However, creep tests performed at 649°C showed a considerable benefit in retaining a level of around 0.2% for each of these elements. An added advantage to a low level of AI + Ti was improved weldability. It is noted that the solubility of aluminium in the alloy had been determined to be about 0.5%.
4. METALLURGICAL STABILITY
Alloy 625 was originally intended to be a solid solution alloy and for the majority of applications it is assumed to behave as one. It is marketed on the basis that its strength is derived from the stiffening effect of molybdenum and niobium on its nickel-chromium matrix; thus precipitation-hardening (age-hardening) treatments are not required.
However, during its development, it was recognised that long-term exposure to intermediate temperatures in the region of 650°C could cause age-hardening effects. These were probably due to the precipitation of gamma prime (γ’), which is an intermetallic phase based on Ni3(Ti, Al) [4]. The exposure times involved were much longer than the 16 hours used in the original screening tests and, whilst this showed that the alloy was age-hardenable, the ageing times required were uneconomically long (i.e. about 200 hours at 649°C). In addition, as discussed below, the presence of γ’ was not really desirable. To improve the metallurgical stability of the alloy, i.e. minimise the risk of age-hardening, the composition was modified by reducing the amount of aluminium and titanium in the alloy to their present amounts.
During the mid to late 1960s, interest was expressed in large-cross-section (200-250 mm diameter) Alloy 625 with minimum yield strength of 552 MPa. One proposal for achieving this was to increase the niobium content to 4% or above. However, Inco preferred to develop the desired properties by control of thermal-mechanical processing and heat-treatment. They believed that a higher-niobium version of Alloy 625 would cause commercial problems, such as duplicate stocking and segmented market demand. In addition, it was thought it would be prone to problems during manufacture, such as increased segregation during melting due to the formation of niobium carbide precipitates; leading to poor malleability and higher scrappage rates.
Earlier laboratory studies had shown that in order to achieve a minimum yield strength of 552 MPa, large sections would require a final thermal processing step. Thermal strengthening was known to be effective but sluggish; occurring in a narrow window around 649°C with the greatest response within 48 hours. Warm work helped the strengthening process, but the inhomogeneous strain likely to occur could result in considerable variation in the properties through the section. An annealing temperature in the range 871-927°C maximum was essential for achieving minimum yield strength of the 414 MPa and an additional ageing heat-treatment at 649°C for 24-48 hours needed to achieve the 552 MPa level.
The gamma prime (γ’) phase has a face centred cubic (FCC) structure and has a window of instability between 600°C and 850°C, inside of which γ' will transform into the hexagonal close packed (HCP) η phase. The formation of γ’ and its subsequent transformation may in part account for the poor creep test results obtained before the levels of aluminium and titanium were tightened. For applications at temperatures below 650 C, the gamma double prime (γ") phase is normally used to strengthen nickel-based superalloys. γ" has a body centred tetragonal (BCT) structure and typically possesses the composition Ni3Nb [5].
In smaller sized specimens (up to 100 mm diameter), the annealing temperature is critical to the ageing response of Alloy 625, with higher annealing temperatures resulting in a poorer response to direct ageing heat treatments. In larger sized specimens (e.g. up to 250 mm diameter), the annealing temperature is less critical. This is because the slower cooling rate from the annealing temperature means the material spends more time in the nucleation temperature range of 732-843°C. The dwell time in this temperature range triggers the precipitation reaction and allows faster growth of subcritical γ” nuclei; thus permitting a normal, though slow, precipitation of γ” particles during the lengthy ageing treatment at 649°C. It would appear that a time of between 2-4 minutes in the critical 760-788°C temperature range is necessary to cause more rapid ageing at 649°C.
If smaller sized specimens are subjected to a high annealing temperature, such as 1149°C, unless an intermediate nucleation treatment of 760°C for 1 hour is performed prior to ageing, an extremely long time is required to produce an ageing reaction at 649°C. A comparison of the ageing times necessary to achieve appreciable hardening is shown in Figure 6. After annealing at 1149°C for 1 hour, in the sample that did not receive a nucleation treatment, no increase in hardness was observed after 96 hours at 649°C; whereas, in the sample that received a nucleation treatment the hardness increased by 11 Rockwell ‘A’ points after ageing at 649°C for 96 hours.
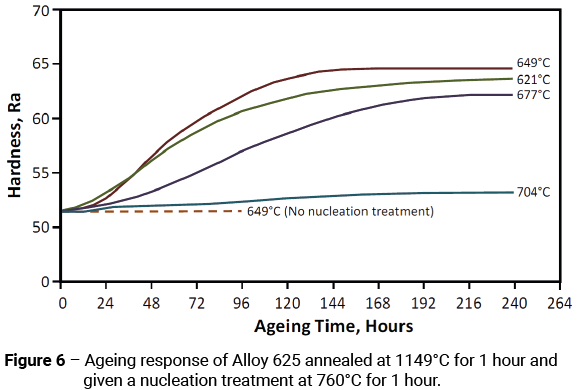
Through control of the aluminium and titanium levels Alloy 625 does not precipitate γ’ (found in most AI-Ti hardened alloys), rather it precipitates a metastable γ” and a stable orthorhombic Ni3Nb phase. The γ” phase confers the most strength to the alloy, and it is the phase of most use in age hardening. The orthorhombic Ni3Nb phase is not coherent with the matrix and contributes to hardening only as a dispersant.
In the solution annealed condition (1093°C minimum), the yield strength of Alloy 625 should be a minimum of 276 MPa. While a minimum room-temperature yield strength of 414 MPa is achievable in cross-sections up to 100 mm and 345 MPa in cross-sections from 100-250 mm, without extensive mechanical working higher minimum values are really only achievable by age hardening for prolonged periods of time, such as 24-48 hrs at 649°C. Figure 7 shows a plot of room-temperature yield strength after exposure to intermediate temperatures for various times up to 1000 hours.
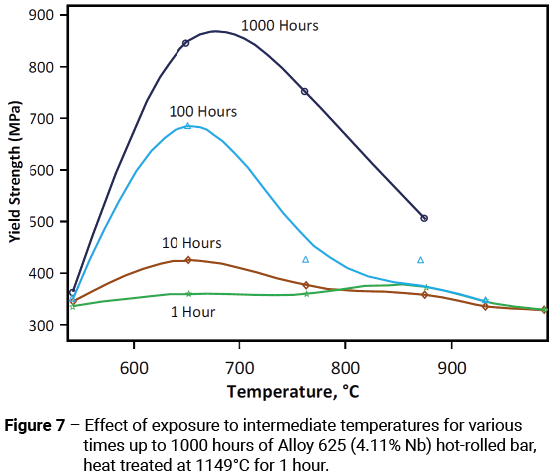
As discussed earlier, controlling the aluminium and titanium content together with that of niobium is important in determining the age-hardening response of the alloy. Higher levels of niobium have a beneficial effect on the response of the alloy to age-hardening, but they can also result in a loss of room-temperature ductility; if the material is subject to prolonged exposure to intermediate-temperatures during service. Under such circumstances, it is likely that the beneficial effects of improved age-hardening response will be more than outweighed by the detrimental effects of the loss in room-temperature ductility.
5. DISCUSSION
As already noted, Alloy 625 is normally considered to be a solid solution alloy. In the oil and gas industry, Alloy 625 is probably used most widely offshore for upstream pipework, where the key features of the material are its strength and corrosion resistance. Given that its strength is so important, it is strange that the age-hardening response of the alloy is not exploited. In fact, in most cases, it is positively discouraged.
This may, in part, be due to the need to ensure compliance with NACE MR0175/ISO 15156-3 [6]. This standard covers the use of materials in H2S containing environments and permits the use of Alloy 625 in any combination of temperature, partial pressure of H2S, chloride concentration and in situ pH occurring in production environments. However, NACE MR0175/ISO 15156-3 considers Alloy 625 to be a solid-solution nickel alloy and only permits its use in the annealed or solution-annealed condition.
The paper by H. L. Eiselstein and D. J. Tillack [2] implies that achieving the strength requirements specified in the relevant standards should not be a problem. However, experience shows that in the case of ‘heavy’ wall fittings and forgings, maintaining the required yield strength and/or tensile strength can be a problem; especially, where the material has to go through a number of hot working operations. It should be noted that the annealing and solution-annealing heat treatments do not improve the strength of the material; if anything, they reduce it.
Given this situation, provided the age-hardening process does not impact on the corrosion performance, toughness and/or ductility of the material, it seems illogical not to take advantage of the age-hardening response of Alloy 625. In the annealed and solution annealed conditions, the effect of the niobium content is largely wasted; it contributes very little to the performance of the alloy unless creep is an issue. However, in the age-hardened condition, its contribution is significant and should be exploited.
References
(1) Stephen Floreen, Gerhard E. Fuchs, and Walter J. Yang, “The Metallurgy of Alloy 625”, Superalloys 718, 625, 706 and Various Derivatives, The Minerals, Metals and Materials Society, 1994.
(2) H. L. Eiselstein and D. J. Tillack, “The Invention and Definition of Alloy 625”, Superalloys 718, 625 and Various Derivatives, The Minerals, Metals and Materials Society, 1991.
(3) H. L. Eiselstein, “Metallurgy of a Columbium-Hardened Nickel-Chromium-Iron Alloy”, Publication 369, ASTM, 1965
(4) Randy Bowman. "Superalloys: A Primer and History". TMS.
(5) Dunand, David C. "Materials Science & Engineering 435: High Temperature Materials". Northwestern University, Evanston. 25 February 2016. Lecture.
(6) NACE MR0175/ISO 15156-3, “Petroleum and natural gas industries – Materials for use in H2S-containing environments in oil and gas production – Part 3: Cracking-resistant CRAs (corrosion resistant alloys) and other alloys”, NACE International, 1440 South Creek Dr., Houston, Texas.