1. INTRODUCTION
Traditionally, hydrogen service has referred to the use of steels at elevated temperatures where damage can occur as a result of atomic hydrogen reacting with carbon present within the steel. This reaction produces methane that can cause blistering and/or fissuring which may result in catastrophic failure of a component. The mechanism and the means of avoiding it are well understood, and generally involve the use of low alloy chrome-moly steels.
With the move towards the use of hydrogen as an alternative to fossil fuels, it is increasingly being handled and stored at ambient temperatures. Whilst the failure mechanisms that occur at elevated temperatures are not an issue there are other factors that need to be considered when deciding on the correct material to select. This technical note considers these factors in relation to the safe use of carbon and carbon-manganese steels.
2. HYDROGEN DAMAGE
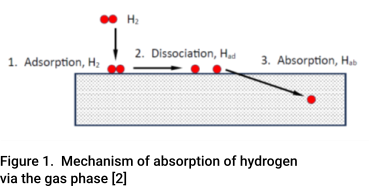
Hydrogen damage is caused by atomic hydrogen absorbed by the steel. It is damaging because it is able to diffuse readily through the steel. At ambient temperatures, atomic hydrogen is most commonly formed as a biproduct of a corrosion reaction. However, it can also form due to the dissociation of molecular hydrogen as indicated in Figure 1.
The solubility of hydrogen in a metal is a function of the partial pressure of hydrogen and increases with increasing temperatures [1]. It is described by Sieverts’s law:
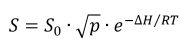
Where S0 is the solubility constant, p the partial pressure, ΔH the heat of solution, R the universal gas constant, and T the absolute temperature in Kelvin [3]. The main thing to recognise from the above equation is that hydrogen solubility is proportional to the square root of the partial pressure of hydrogen in the gas/liquid.
Where atomic hydrogen forms as a result of a corrosion reaction, it is generally not a problem as the partial pressure is low and it readily combines to form molecular hydrogen so is not absorbed by the steel. However, if hydrogen sulphide (H2S) is present in any significant quantity, this can poison the reaction and allow atomic hydrogen to easily permeate the steel.
In systems used to store and/or transport hydrogen, particularly at high pressure. Hydrogen solubility becomes a big problem. Whilst molecular hydrogen itself is not an issue as the molecules are too big to pass between the metal atoms in the steel, as indicated in Figure 1, hydrogen can dissociate.
In an ideal world, the hydrogen absorbed by the steel will permeate through the structure and eventually escape to the atmosphere. However, there are discontinuities present in the steel, primarily inclusions. The hydrogen will become trapped at these locations where it will recombine to form molecular hydrogen generating an enormous internal pressure.
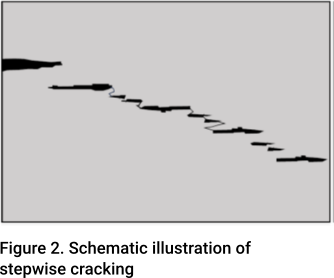
If the inclusions are planar in shape, tiny cracks can form at the ends of the inclusion. If the steel is ‘dirty’, i.e. there is a high density of inclusions, the cracks can coalesce resulting in a phenomenon known as stepwise cracking, commonly referred to as hydrogen-induced cracking (HIC). This is illustrated in Figure 2. Where this occurs, the material will be irreparably damaged.
The problem of stepwise cracking and issue of fracture toughness in general is exacerbated by the embrittling effect hydrogen has on the steel. When hydrogen is present, the steel will exhibit reduced ductility and fracture toughness, which can result in other failure mechanisms depending on the microstructure of the steel.
These mechanisms are commonly referred to as hydrogen stress cracking (HSC) and result from the presence of hydrogen in the metal together with tensile stress (residual and/or applied).
3. SOUR SERVICE
As noted above, hydrogen damage is normally most pronounced where corrosion occurs in the presence of H2S. The consequences of the sudden failure of a metallic component associated with its exposure to an H2S-containing production fluid led to the preparation of the first edition of NACE MR0175; published in 1975 by the National Association of Corrosion Engineers.
NACE MR0175 deals primarily with the phenomenon of sulphide stress corrosion cracking (SSC) and provides guidance for the selection and specification of SSC-resistant materials. It mentions the phenomena of HIC and HSC, as well as other mechanisms that can occur, namely soft-zone cracking (SZC) and stress-orientated hydrogen-induced cracking (SOHIC).
Under service loads, soft zones can yield and accumulate plastic strain locally, increasing the susceptibility to cracking of an otherwise SSC-resistant material. Such soft zones are typically associated with the heat affected zones of welds in carbon steels [4]. Figure 3 illustrates a typical soft-zone crack.

With SOHIC, staggered small cracks form approximately perpendicular to the principal stress (which again can be residual and/or applied).
These result in a “ladder-like” crack array linking (sometimes small) pre-existing HIC cracks, as illustrated in Figure 4. SOHIC is a relatively uncommon phenomenon and is usually associated with low-strength ferritic pipe and pressure vessel steels.
4. MATERIAL CONSIDERATIONS
Whilst it is highly unlikely that SSC would ever occur in plant being used to transport and/or store hydrogen (given that the gas should be dry and there should be no H2S present), the recommendations of NACE MR0175 can be applied to ensure the avoidance of hydrogen damage in such plant.
NACE MR0175 applies to the qualification and selection of materials for equipment designed and constructed using load-controlled design methods, e.g. ASME B31.3 and ASME VIII Divisions 1 and 2. The recommendations of NACE MR0175 may not be applicable to equipment designed using strain-based design methods, e.g. elastic-plastic analysis.
The two principal factors governing a material’s resistance to hydrogen damage are microstructure and chemical composition.
4.1. HIC RESISTANCE
In relation to HIC resistance, microstructure and chemical composition are equally important; specifically, the inclusion content. Non-metallic inclusions in carbon steels are the result of tramp elements introduced during the steelmaking process. Manganese sulphide (MnS) is arguably the most important non-metallic inclusion in relation to HIC. It is not only the volume fraction of MnS inclusions that is important, but also their shape.
The volume fraction of MnS inclusions is a function of the sulphur content; the lower the sulphur (S) content, the smaller the volume fraction of MnS inclusions. The shape of the MnS inclusions depends on how the steel is worked after it has been cast. MnS inclusions are relatively soft in relation to the steel matrix. Rolling for example elongates and flattens the inclusions.
The presence of elongated inclusions results in anisotropy of toughness and ductility properties as well as a significant reduction in the HIC resistance of the steel. Calcium treatment is often used when HIC resistance is required. It is carried out during the steelmaking process and involves adding calcium to the melt after the steel has been killed (deoxidised).
Killed steel is steel that has been completely deoxidized by the addition of an agent before casting such that there is practically no evolution of gas during solidification. Aluminium is the most widely used element for the deoxidation of steel. It reacts with oxygen in the steel to produce aluminium oxide (Al2O3). Most of the Al2O3 produced partitions to the slag produced during deoxidation treatment. The slag is skimmed off, thereby removing the dissolved oxygen from the liquid steel bath.
Calcium (Ca) reacts with residual Al2O3 in the melt and forms liquid calcium-aluminate. It also reacts with sulphur (S) to form calcium sulphide (CaS). This dissolves and/or precipitates on calcium-aluminates to form complex Ca-Al-O-S globular type inclusions. Any MnS present will also tend to precipitate on these inclusions to produce bull-eye shaped inclusions. These are less dense than the liquid metal, so float up into the slag.
Any inclusions left in the steel after solidification are hard and do not flatten out during subsequent working of the steel. This change in composition and mode of precipitation of sulphide inclusions during steelmaking is known as sulphide shape control. The calcium to sulphur ratio is an important indicator of the cleanliness of a steel. The optimum calcium to sulphur ratio is approximately two to one.
4.2. HYDROGEN STRESS CRACKING
The risk of hydrogen stress cracking (HSC) in steels is greatest at around 20°C and reduces with increasing temperature. Steels are relatively immune to HSC at temperatures above 150°C. As its name suggests, HSC requires the presence of both hydrogen (atomic) and a tensile stress (applied and/or residual).
HSC is the result of hydrogen embrittlement. This is a complex process involving several distinct contributing micro-mechanisms, not all of which need to be present. In steels, the principle mechanisms are the enhanced decohesion at internal surfaces and localised plasticity at crack tips, which assist in the propagation of cracks.
- Hydrogen increases the nucleation and movement of dislocations at a crack tip. This mechanism is known as hydrogen enhanced localised plasticity (HELP) and results in crack propagation by localised ductile failure at the crack tip with less deformation occurring in the surrounding material, which gives a brittle appearance to the fracture [5].
- Hydrogen decreases the emission of dislocations at the crack tip, producing a ductile-to-brittle transition. This prevents plastic deformation at the crack tip leaving a ‘sharp’ crack that can result in brittle failure [6].
- Interstitial hydrogen reduces the stress needed for metal atoms to pull apart. This mechanism is known as hydrogen enhanced decohesion (HEDE) and can only occur when the local concentration of hydrogen is high, for example due to the increased hydrogen solubility in the tensile stress field at a crack tip or at stress concentrators.
Fracture toughness decreases with increasing strength. Consequently, higher-strength steels are more susceptible to hydrogen embrittlement. As with sour service, the selection of steels with a yield strength less than or equal to 355MPa, will significantly reduce the risk of hydrogen stress cracking.
4.3. STRESS-ORIENTATED HYDROGEN INDUCED CRACKING
Stress orientated hydrogen induced cracking (SOHIC) is a form of through thickness cracking generally associated with sulphide stress cracking (SSC). It occurs primarily in rolled and forged products. It is different to HIC as it requires the presence of both hydrogen and an applied stress. It occurs when clusters of small planar cracks, aligned normal to the rolling/forging direction, coalesce to form a significant crack normal to the direction of the applied stress.
To minimise the risk of SOHIC, normal practice is to select steels considered to be resistant to HIC. However, unlike HIC, the susceptibility to SOHIC does not appear to depend on the presence of inclusions. Work by the Materials Properties Council suggests that HIC resistant steels may be more susceptible to SOHIC [7].
The fact that SOHIC occurs in the absence of inclusions implies that the clusters of planar cracks are initiating at interfaces that are stronger than the interface between an inclusion and the surrounding matrix. Microstructural inhomogeneities, such as ferrite-pearlite interfaces, for example in heavily banded steel, could provide such initiation sites. However, for this to occur, high stress and significant hydrogen charging would be necessary.
4.4. SOFT-ZONE CRACKING
Soft-zone cracking (SZC) is a specific form of SOHIC and occurs exclusively in the softened heat affected zone (HAZ) of weldments. The width of the soft zone of the HAZ is very narrow. It is constrained by surrounding hard zones which prevent the material within the soft zone from deforming. Because of this inability to deform, combined with effect of hydrogen on the cohesive strength, the material ruptures producing micro-fissures which form parallel to the direction of the applied stress. Localised yielding in the slip-bands connecting these fissures results in further rupturing of the material, eventually producing a through- thickness crack characteristic of SZC. The situation is worst at the mid-thickness of the weldment, so any cracks tend originate here and propagate out towards the surfaces.
SZC is a particular problem with linepipe steels produced by thermo-mechanically controlled processing (TMCP) where the carbon content is low to achieve high toughness and good weldability. The low carbon content results in a softened HAZ due the weld thermal cycle. SZC is less of an issue with normalised, normalised and tempered, and quenched and tempered steels, where both the carbon content and carbon equivalent are significantly higher.
5. DISCUSSION
If it is assumed that the steel will become saturated with hydrogen during service, then the only means to reduce the risk of hydrogen damage are to control the microstructure of the steel and to limit the stresses acting on it.
5.1. STRESS
Limiting the stresses acting on a component is a mechanical consideration. Design codes specify an allowable stress beyond which the material may not to be loaded. The maximum allowable stress for process piping will generally be around two-thirds the yield strength of the material, although often a lot less. However, if there are welds present that have not been post weld heat treated, the residual stresses are likely to be a lot higher than the allowable stress, possibly close to the yield strength.
Post weld heat treatment (PWHT) will certainly help mitigate the effects of hydrogen embrittlement. It will do nothing to reduce the risk of stepwise cracking, which is purely down to the microstructure of the steel. The influence of PWHT on the susceptibility of a material to SOHIC is not clear, but it is generally considered to be beneficial. However, where SZC is a concern, it is doubtful that PWHT will have much if any effect on the susceptibility of the material as it is unlikely to eliminate the soft zone.
5.2. MICROSTRUCTURE
Controlling the microstructure of the steel is crucial to minimising the risk of hydrogen damage. The risk of stepwise cracking can be eliminated by using a clean steel that has been calcium-treated. This should also reduce the risk of SOHIC, although there is some debate over the effects of steel type and quality on the risk of SOHIC.
In terms of defining what constitutes a 'clean' and/or a 'sour service' steel, four steel types have been identified: [4]
5.2.1. Conventional Steel
This is commercially produced and is either hot-finished or normalised. It can have relatively high levels of impurities, notably sulphur and phosphorus. The sulphur content can be as high as 0.040 wt%, although this would be unusual. In plate form, it is likely to have very little resistance to HIC even under moderate exposure conditions.
In the European market, the levels of impurities are more tightly controlled to improve the mechanical properties, principally toughness. Typically, the sulphur content will be in the range 0.005-0.015 wt%, depending on the product form.
5.2.2. Low Sulphur Conventional Steel
This is commercially produced. It has a lower sulphur content than a conventional steel, typically in the range 0.003 to 0.010 wt%. It should exhibit improved mechanical properties in comparison to a conventional steel, principally toughness. These steels are not normally processed to provide specific HIC resistance so can still be significantly susceptible to HIC even in moderate service environments.
In the European market, the maximum sulphur content of a 'low sulphur' steel will typically be around 0.008 wt%. Calcium treatment may also be carried out to control the inclusion content and shape. Again, this is to ensure good toughness and good through thickness properties in plate rather than to improve HIC resistance.
5.2.3. HIC-Resistant Steel
This is essentially a conventional steel that has been metallurgically processed to enhance its HIC resistance. HIC-resistant steels are characterised by an ultra-low sulphur content, typically below 0.003 wt%. They may have been calcium-treated to provide sulphide shape control. As a minimum they will have been normalised to refine their microstructure. Whilst they have much improved resistance to HIC in comparison to conventional steels, they may still show some degree of susceptibility to HIC and SOHIC in severe service environments.
In the European market, linepipe steels intended for sour service typically have a sulphur content of 0.003 wt% or less. They also have restricted levels of manganese and phosphorus. They are almost certainly calcium treated and will normally be supplied in the TMCP or quenched and tempered condition. As noted above, with TMCP the carbon content is very low, typically around 0.04 wt%, as such, whilst resistant to HIC, they are susceptible to SOHIC, specifically SZC.
5.2.4. Ultra-Low Sulphur Advanced Steels
These steels are made by the most modern steel making and processing techniques. Typically, they have an ultra-low sulphur content (S ≤ 0.002 wt%). They have low carbon equivalents and a reduced carbon content in comparison to conventional steels of comparable tensile strength. They are produced by TMCP and/or accelerated cooling techniques. They have very fine-grained ferritic or ferritic/bainitic microstructures with little or no microstructural banding. They have exceptional resistance to HIC and good resistance to SOHIC even in severe service environments.
5.3. HYDROGEN EMBRITTLEMENT
Hydrogen embrittlement is unavoidable if atomic (diffusible) hydrogen is present. Even if precautions are taken to avoid hydrogen damage, the fracture toughness and ductility of the steel will be reduced. This is evidenced in work by Somerday [8]. This showed that whilst hydrogen has little effect on either the yield or tensile strength, it has a significant effect on the elongation and the reduction-in area of both smooth and notched tensile specimens (the reduction in area decreased on average by 37% in smooth samples and 71% in notched samples). It was also observed that the fracture toughness decreased significantly. Figure 5 shows the dependence of the fracture toughness on hydrogen pressure for two low strength carbon steels (yield strength 280-375 MPa) [8].

This drop-off in fracture toughness and ductility can be countered by specifying higher absorbed energy criteria for impact testing and higher elongation and reduction-of-area for tensile testing.
As already discussed, improved toughness and ductility can be obtained by using a clean steel that has been calcium treatment. Toughness and ductility are also dependent on the grain structure. A fine-grained structure should produce good toughness and ductility.
Fine grain practice is a steelmaking practice for other than stainless steel that is intended to produce a killed steel that can meet the requirements specified for fine austenitic grain size. It normally involves the addition of one or more austenitic grain refining elements in amounts that have been established by the steel producer as being sufficient. Austenitic grain refining elements include, but are not limited to, aluminium, niobium, titanium, and vanadium.
The sulphur content should be kept to a minimum (ideally to a maximum of 0.005 wt% for rolled products and 0.010 wt% for forgings). As noted above, the steel should be produced to fine grain practice. It should also be calcium treated to control the shape of any inclusions present.

As might be expected, the risk of hydrogen damage increases with strength. This is indicated in Figure 6, where within the pink zone, materials are susceptible to hydrogen cracking under constant load. Above this zone, they will almost certainly fail. Below this zone they should be relatively immune to hydrogen damage.
If possible low to medium strength steels should be used. For process pipework, ASTM A333 Grade 6, ASTM A350 Grade LF2, and ASTM A420 Grade WPL6, or equivalent, are recommended. For pipeline applications, as a maximum API 5L Grade X60, MSS SP-44 Grade F60, and MSS SP-75 Grade WPHY60, should be used (ideally the strength should be limited to Grade X52).
In all cases, the steel should be supplied in the normalised, normalised and tempered, quenched and tempered, or TMCP condition. The better the quality (i.e. grain structure and cleanliness), the more resistant the steel will be to hydrogen damage.
REFERENCES
- Sieverts, A.; Krumbhaar, W.: “Über die Löslichkeit von Gasen in Metallen und Legierungen”, Berichte der deutschen chemischen Gesellschaft, 43 (1910), pp 893–900
- Rehrl, J.: Wasserstoffversprödung in hochfesten, mikrolegierten Stählen, Dissertation, TU München, 2013
- Rawls, G. B.; Adams, T.: “Hydrogen production and containment”, in: Somerday, B. P.; Gangloff, R. P. (Eds.): Gaseous hydrogen embrittlement of materials in energy technologies: Volume 1: The problem, its characterisation and effects on particular alloy classes, Cambridge: Woodhead Publishing Ltd, 2012
- NACE MR0175/ISO 15156-1, “Petroleum and natural gas industries-Materials for use in H2S-containing Environments in oil and gas production-Part 1. General principles for selection of cracking-resistant materials”, ISBN 1-57590-1765
- Haiyang Yu (February 2009). "Discrete dislocation plasticity HELPs understand hydrogen effects in bcc materials". Journal of the Mechanics and Physics of Solids. 123: 41–60.
- Song, Jun (11 November 2012). "Atomic mechanism and prediction of hydrogen embrittlement in iron". Nature Materials. 12 (2): 145–151.
- Cayard M S, Kane R D and Horvath R J. “SOHIC resistance of C-Mn plate steels used in refinery service”. NACE Corrosion/2002, Denver, Colorado, USA, 7-11 April 2002. Paper 02554.
- Somerday, B.P. 2007. Technical reference on hydrogen compatibility of materials. Carbon steels: C-Mn alloys (code 1100). Sandia National Laboratories