1. Introduction
Hydrogen is viewed as a potential solution to the environmental issues associated with our reliance on fossil fuels for energy needs. To this end, considerable effort is being expended in all areas of the hydrogen economy; in particular, hydrogen production, transportation, and storage, as well as the means of converting hydrogen into useful forms of energy, e.g. fuel cells.
Achieving a viable hydrogen economy will not be easy. The costs of producing hydrogen from renewable sources are still relatively high. There are physical issues with its transportation and storage, due to the low energy density of hydrogen gas. The cost and reliability of fuel cells is a further consideration.
The production, transportation, and storage of hydrogen are not without their challenges. Probably the greatest of these is hydrogen embrittlement. There are three distinct types of hydrogen embrittlement. These are environmental hydrogen embrittlement, internal hydrogen embrittlement, and hydrogen-reaction embrittlement.
As mentioned above, hydrogen gas has a low energy density. Because of this, a large volume is needed to provide the necessary energy requirements. One way to increase the energy density is to reduce the volume by liquefying the hydrogen. However, this is energy intensive and uses approximately 30% of the energy content of the hydrogen. It is all only possible at very low temperatures which affect the mechanical properties of the materials used.
A less energy intensive way to increase the energy density is to increase the pressure at which it is stored and/or transported, but this too presents a number of challenges; not least, safety. Hydrogen is prone to leak through very small gaps and permeate through certain materials. There is also the issue of hydrogen embrittlement. Increasing the pressure increases the risk of leakage and the damaging effects of hydrogen embrittlement.
Proper attention to design and appropriate material selection is vital. When selecting materials for use with liquid hydrogen it must be remembered that the plant and equipment may also operate at higher temperatures; so hydrogen embrittlement is always a concern. There are a number of ways that susceptibility to hydrogen embrittlement can be reduced both from a design and materials viewpoint.
Various national and international codes and standards have been developed that cover the design, manufacture, and operation of plant, equipment, and pipelines for the production, storage, and transportation of hydrogen. This article aims to provide an overview of the issues associated with the production, storage, and transportation of hydrogen and how these are addressed in the various national and international codes and standards.
2. Hydrogen Production
Hydrogen is used in a wide variety of industrial applications. Its two biggest uses are in fossil fuel processing (e.g., hydrocracking) and ammonia production (mainly for the fertiliser market). For this reason, most industrial hydrogen is currently produced by thermochemical processes. However, it can also be produced using electrolytic and photolytic methods, which offer a much greener alternative to some of the thermochemical processes.
Feedstocks can be both non-renewable (i.e. fossil fuels) and renewable (e.g. biomass), but currently they tend to be fossil fuels (primarily methane).
2.1. Thermochemical
The main thermochemical processes are steam reforming, autothermal reforming, and gasification. As well as hydrogen, other gases are produced; primarily, carbon monoxide. To further enhance the amount of hydrogen produced, a water-gas shift reactor is often used. Such reactors convert a portion of the carbon monoxide in the produced gas (syngas) to carbon dioxide and additional hydrogen, via the water gas shift reaction.
2.1.1. Steam Reforming
Steam reforming is probably the most widely used of the thermochemical processes. It is an endothermic equilibrium reaction, in which hydrogen is generated through a catalysed reaction between a hydrocarbon and steam. The reaction is reversible and can be best described as:
CnHm + n H2O ⇌ n CO + (n + ½m) H2
‘n’ and ‘m’ represent the number of carbon and hydrogen atoms, respectively. ‘m’ is equal to two times ‘n’ plus two. When ‘n’ is equal to one and ‘m’ is equal to four, CnHm is CH4, i.e. methane; when ‘n’ is equal to two and ‘m’ is equal to six, CnHm is C2H6, i.e. ethane; and so on.
Hydrogen produced by this method is commonly referred to as grey or blue. The difference between grey and blue hydrogen is that, with grey hydrogen, the carbon dioxide produced is released to the atmosphere; with blue hydrogen, the carbon dioxide is captured.
Of the various reforming processes, because of its availability, methane steam reforming is the most viable means of generating hydrogen. Methane is reacted with steam at 700-1000°C at a pressure of 3-25 bar. In addition to carbon monoxide and hydrogen, the syngas will contain unreacted methane and carbon dioxide, therefore, further processing is required to obtain pure hydrogen.
From a design/materials selection viewpoint, the main issues are the high operating temperature and the potential for hydrogen reaction embrittlement. The high operating temperature will not only impact on the creep resistance of the material but, combined with the syngas, will also hugely increase the risk of hydrogen-reaction embrittlement and oxidation.
2.1.2. Autothermal Reforming
Autothermal reforming is similar to methane steam reforming but uses oxygen, and carbon dioxide or steam in a reaction with methane to form syngas. In essence, it is a combination of both steam reforming (endothermic) and partial oxidation (exothermic) reactions. The overall reaction is exothermic so has the advantages of not requiring an external heat source.
Autothermal reforming takes place in a single chamber, where the methane is partially oxidised. It is simpler and less expensive than steam reforming of methane. When carbon dioxide is used, the hydrogen to carbon monoxide ratio in the syngas is approximately 1:1. When steam is used, the hydrogen to carbon monoxide ratio produced is approximately 2.5:1. The outlet temperature of the syngas is between 950-1100°C. The outlet pressure can be as high as 100 bar.
Again the main issues from a design/materials selection viewpoint are the high operating temperature and the potential for hydrogen reaction embrittlement.
2.1.3. Gasification
In the gasification process, carbonaceous materials are converted to syngas typically at 700-1000°C, without combustion, in the presence of an oxidising agent; normally, oxygen or steam. The amount of hydrogen produced is dictated by the process conditions, the temperature of the steam flow, and the nature of the feedstock.
The feedstock needs to go through a number of processes before gasification can occur. These all take place within the gasifier. First, there is the dehydration or drying process, which occurs at around 100°C. This is followed by pyrolysis at 200-300°C, during which volatiles are released and the char which will undergo the gasification reaction is produced. Oxygen is then added resulting in combustion of the volatiles together with some of the char. This provides the heat necessary for the subsequent gasification reactions.
Gasification occurs when steam is injected. The char reacts with the steam and any carbon dioxide carried over from the combustion process to produce syngas. If we use “C” to represent the char, the gasification process can best be described by the reactions
C + H2O ⇌ H2 + CO
C + CO2 → 2CO
In addition to the above reactions, the reversible water-gas shift reaction also occurs and reaches equilibrium very fast at the temperatures in a gasifier. This balances the concentrations of carbon monoxide, steam, carbon dioxide and hydrogen.
The two most common forms of hydrogen produced by this method are normally referred to as brown or black hydrogen depending on the type of coal used in their production. This method of hydrogen production is not environmentally friendly as the waste gases are generally released to the atmosphere.
The main issues from a design/materials selection viewpoint with a gasifier are not only the high temperature at which the gasification reaction occurs, which also raises concerns regarding hydrogen reaction embrittlement, but the significant difference in temperature between the inlet and outlet streams. The nature of the feedstock and the possible need to dry it before use, also mean that corrosion is a major consideration.
2.1.4. Water-Gas Shift Reaction
The water-gas shift reaction is commonly used to enhance the hydrogen to carbon monoxide ratio in the syngas. The reaction is exothermic so, as in the case of autothermal reforming, does not require an external heat source.
In the production of hydrogen, it is used to convert the carbon monoxide present in the syngas to carbon dioxide and, in the process, maximise the amount of hydrogen produced.
CO + H2O ⇌ CO2 + H2
The water-gas shift reaction is favoured at lower temperatures and higher steam content.
There are four types of water-gas shift reactor in common use. The type of reactor actually used depends on nature and condition of the syngas:
- High Temperature Shift Conversion (HTSC) – this is used when the syngas does not contain significant amounts of sulphur. Typically, the syngas enters the reactor at 300-400°C and passes through a bed of copper promoted iron-chromium catalyst to promote the reaction.
- Medium Temperature Shift Conversion (MTSC) – again, this is used when the syngas does not contain significant amounts of sulphur. Typically, the syngas enters the reactor at 150-300°C and passes through a bed of copper-zinc-alumina catalyst to promote the reaction.
- Low Temperature Shift Conversion (LTCS) – this is often used downstream of a HTSC reactor. Typically, the syngas enters the LTSC reactor at 175-250°C. A zinc-copper catalyst is commonly used. For this reason, it is important to control the sulphur and chloride content of the reactants as these can poison the catalyst.
- Sour Gas Shift Conversion (SGS) – this is used when the syngas contains appreciable levels of hydrogen sulphide and is typically used in gasification processes where the feedstock is not desulphurised prior to entry into the gasifier. Typically, the syngas enters the reactor at 230-260°C where it passes through a bed of cobalt-molybdenum catalyst.
The design/materials selection issues with water-gas shift reactors are slightly different to other types of reactors. Because of the relatively low operating temperatures, creep and hydrogen reaction embrittlement are less of a concern. The principal concerns are with environmental hydrogen embrittlement, internal hydrogen embrittlement, and corrosion (particularly sour service corrosion where the syngas contains appreciable levels of hydrogen sulphide).
2.1.5. Methane Pyrolysis
Methane pyrolysis as a means of hydrogen production is a recent "no greenhouse gas" one-stage process. It involves bubbling methane through a molten metal catalyst at high temperature (approximately 1065°C). It has the potential to produce high volumes of hydrogen gas at relatively low cost. A by-product of the reaction is solid carbon (C), which could be used in steel production as an alternative to coal. No greenhouse gases are released in the reaction.
CH4 (gas) → C (solid) + 2 H2 (gas)
This form of hydrogen is commonly referred to as turquoise hydrogen. As noted above, it can be considered environmentally sound as no ‘waste’ gases are produced.
Like other thermochemical processes, the main design/materials selection issues with methane pyrolysis are the high temperature at which the reaction occurs and the potential for hydrogen reaction embrittlement.
2.2. Electrolytic
Due to their low input energy requirements and high conversion efficiency, when compared to thermochemical methods, electrolytic methods (electrolysis) represent a promising alternative to carbon-based hydrogen production. Electrolysis uses electricity to split water into hydrogen and oxygen.
Hydrogen produced by electrolysis is commonly referred to as purple/pink, yellow, or green depending on the source of electricity. Purple/pink hydrogen is produced using electricity from nuclear power, yellow hydrogen is produced using electricity specifically from solar power, and green hydrogen is produced from electricity from renewable sources (i.e. wind, solar, tidal, etc.)
Electrolysis takes place in a unit known as an electrolyser. The principal components of an electrolyser are an electrolyte and electrodes, i.e. an anode and a cathode. For carbon-free production, the source of the electricity could be renewable or nuclear. There are different types of electrolysers, which function in different ways.
2.2.1. Polymer Electrolyte Membrane Electrolyser
In a polymer electrolyte membrane (PEM) electrolyser, the electrolyte is a solid specialty plastic material. Water reacts at the anode to form oxygen and positively charged hydrogen ions. The hydrogen ions move across the membrane to the cathode where they combine with electrons from the external power source to form hydrogen gas.
The reaction at the anode is:
2 H2O → O2 + 4 H+ + 4 e–
The reaction at the cathode is:
4 H+ + 4 e– → 2 H2
PEM electrolysers typically operate at 70-90°C. Ignoring the electrodes and electrolyte, which should be considered as consumables, the principal design/materials selection issues with a PEM electrolyser are corrosion and the risk of both environmental hydrogen embrittlement and internal hydrogen embrittlement.
2.2.2. Alkaline Electrolyte Membrane Electrolyser
In an alkaline electrolyte membrane (AEM) electrolyser, hydrogen is formed together with hydroxide ions (OH–) at the cathode. The hydroxide ions are transported through the electrolyte to the anode where they combine and give up their extra electrons to produce water and oxygen.
The reaction at the cathode is:
4 H2O + 4 e– → 2 H2 + 4 OH–
The reaction at the anode is:
4 OH– → 2 H2O +O2+ 4 e–
AEM electrolysers offer a number of advantages over PEM electrolysers. The electrode (catalyst) materials are significantly cheaper. There is less dissolution of anodic catalyst. AEM electrolysers are more durable due to an exchangeable electrolyte. The purity of the gases produced is higher due to lower gas diffusivity in alkaline electrolyte.
AEM electrolysers typically operate at below 100°C. Again, ignoring the electrodes and electrolyte, which should be considered as consumables, the principal design/materials selection issues with an AEM electrolyser are corrosion and the risk of both environmental hydrogen embrittlement and internal hydrogen embrittlement.
2.2.3. Solid Oxide Electrolyser
Solid oxide electrolysers generate hydrogen in a slightly different way to PEM and AEM electrolysers. Solid oxide electrolysers use a solid ceramic material as the electrolyte. For the “electrolyte” to function properly, solid oxide electrolysers must operate at 500-850°C. Steam at the cathode combines with electrons from the external power source to form hydrogen gas and negatively charged oxygen ions (O2–). The oxygen ions pass through the solid ceramic membrane and react at the anode to form oxygen gas.
The reaction at the cathode is:
2 H2O + 4 e– → 2 H2 + 2 O2–
The reaction at the anode is:
2 O2– → O2+ 4 e–
Because of the high temperatures at which solid oxide electrolysers must operate, the design/materials selection issues are primarily creep, loss of mechanical strength, and the potential for hydrogen reaction embrittlement.
2.3. Photolytic
Photolytic hydrogen production methods utilise photocatalysis to split water into hydrogen and oxygen using the energy from sunlight. In theory, only light energy (photons), water, and a catalyst are required. The technology is still in its infancy and the hydrogen production efficiency is very low. Emerging direct water-splitting technologies are being developed. These include photobiological systems, which take advantage of the fact that some microbes produce hydrogen in their metabolic activities using light energy, and photoelectrochemical systems, which are engineered to increase the hydrogen production efficiency.
Photolytic hydrogen production methods are highly endothermic process. Because it requires large amounts of input energy, it is incompatible with existing energy generation. For this reason, it is unlikely to replace the more established methods of hydrogen gas; certainly, in the near future.
3. Hydrogen Storage
Hydrogen storage is key to enabling the advancement of hydrogen and fuel cell technologies. Because of its low energy density, advanced storage methods that have potential to increase its energy density are required.Traditionally, hydrogen has been stored as either a gas or a liquid. It is most commonly stored as a gas, because as a liquid it must be stored at minus 253°C. Figure 1 shows the phase diagram for molecular hydrogen H2. It indicates that hydrogen only exists as a liquid between the solidus line and the line from the triple point at minus 253°C (21.2 K) and the critical point at minus 240°C (33 K).
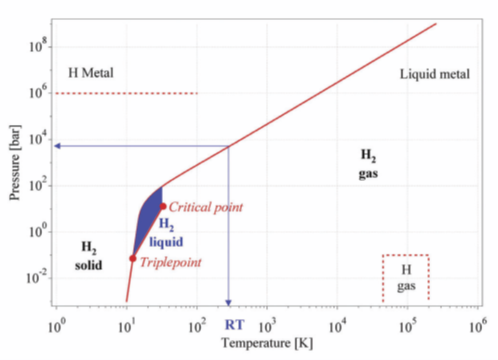
Figure 1. Primitive phase diagram for hydrogen [1]
Interest in using hydrogen for
on-board storage of energy in zero-emissions vehicles is driving the
development of new methods of storage (Gas compression and liquefaction both require
significant amounts of energy). Some of
these methods take advantage that hydrogen can also be stored on the surfaces
of solids (by adsorption) or within solids (by absorption).
3.1. Compressed hydrogen
Compressed hydrogen is used in hydrogen pipeline transport and in compressed hydrogen tube trailer transport. Compressed hydrogen is most commonly stored in high pressure gas cylinders at pressures up to 200 bar. The ideal material for such cylinders has a high tensile strength, a low density, and does not react with hydrogen or allow hydrogen to diffuse into it. The materials most commonly used to date are austenitic stainless steel, copper or aluminium alloys as these are largely immune to hydrogen effects at ambient temperatures.
3.2. Liquid hydrogen
To exist as a liquid, hydrogen must be cooled below its critical point of minus 240°C. However, for it to be in a fully liquid state at atmospheric pressure, it needs to be cooled to approximately minus 253°C. Liquefaction is achieved by successive compression, cooling, and expansion of the gas, e.g. the Joule-Thompson cycle (Linde cycle). As noted above, a drawback with liquid hydrogen storage is the energy required to liquefy the gas; this can equal almost half the combustion energy of the hydrogen. Another drawback is boil-off losses from the liquid storage vessel as a result of heat leaks. The magnitude of these losses is a function of the size, shape, and thermal insulation of the vessel. They are proportional to the surface area-to-volume ratio, such that the evaporation rate diminishes as the size of the storage tank increases.
To avoid issues with cold embrittlement, storage facilities operating at cryogenic temperatures will normally be constructed of materials that do not exhibit a ductile-brittle transition, e.g. austenitic stainless steel, and most alloys of aluminium.
3.3. Ammonia
Ammonia can be used as a fuel or as a means of transporting hydrogen through synthesis and subsequent ammonia cracking (decomposition).
In comparison to other hydrogen storage materials, ammonia (NH3) has the advantages of a high hydrogen density, easy catalytic decomposition, and established technologies for its synthesis and distribution. Ammonia has the potential to replace carbon dioxide producing fuels in various forms of transportation. Like hydrogen, it produces only water and nitrogen as combustion products when burned in internal combustion engines, but the safety and logistic problems of using hydrogen fuels in motor vehicles are avoided.
Ammonia has an octane rating approximately 50% higher than that of gasoline but has a relatively low energy density per unit volume (about half that of gasoline). Its physical properties closely resemble those of propane, in that it can be stored and transported as a liquid under moderate pressure at ambient temperatures. It is widely used as a fertiliser so that facilities for its storage, handling, and transportation are already available.
Toxicity is a concern in relation to the use of ammonia as a fuel, but it does not directly affect material selection, which is dependent on the operating temperature. Whilst normal carbon steel can be used at ambient temperature, special steels are required at low temperatures to avoid embrittlement. Impurities in liquid ammonia such as air or carbon dioxide can cause stress corrosion cracking of mild steel. Trace amounts of ammonia in the hydrogen after decomposition are also a concern. Ammonia is highly corrosive towards copper and zinc.
3.4. Metal Hydrides
An alternative method of hydrogen storage is as a metal hydride in the solid state; particularly, in relation to hydrogen-fuelled road vehicles. Various metals and alloys absorb hydrogen reversibly to form metal hydrides so can be used as “carriers”. Metal hydrides are classified as A, A2B, AB, AB2, and AB5, where metal A is an early transition metal (e.g., titanium, vanadium), a rare-earth metal or magnesium, and B is aluminium, chromium, cobalt, iron, nickel, or manganese. The most important factor in relation to the practical use of metal hydrides is the ability of the “carrier” to absorb and release the hydrogen multiple times without deterioration.
Hydrogen is stored when it dissociates into atomic hydrogen at the surface of the “carrier” and is absorbed. The absorbed hydrogen atoms then diffuse into the “carrier” where they can take the form of a random solid solution or an ordered hydride structure. The quantity of hydrogen absorbed is expressed in terms of hydride composition, either on a molar or a weight-percent basis. Volumetrically, the hydrogen content of the “carrier” may be as high as that in liquid hydrogen.
The range of composition in some metal hydrides is quite wide (i.e., they exhibit a variable metal-to-hydrogen ratio), whereas in others it is fairly narrow. The absorption process is generally exothermic; therefore, in order to absorb hydrogen continuously to the maximum capacity, heat must be removed. The direction of the hydrogen absorption-desorption process is determined by the pressure of the hydrogen gas. If the pressure is above the equilibrium value, then the hydride will be formed. Conversely, below the equilibrium pressure, hydrogen is released and the metal/alloy returns to its original state.
The equilibrium pressure itself depends on temperature; it increases with increasing temperature and decreases with decreasing temperature. Depending on their hydrogen absorption-desorption characteristics, metal hydrides can be broadly categorised as high-temperature, medium-temperature, and low-temperature. Of these, the low temperature category is probably the most useful as hydrogen storage can be achieved close to ambient temperature; particularly, when used to supply hydrogen to a fuel cell. In this case, the heat required for hydrogen desorption would be provided by the waste heat from the fuel cell.
3.5. Chemical and Related Storage
Organic liquids such as cyclohexane, can be used as chemical “carriers” of hydrogen. In the case of cyclohexane the hydrogenation and dehydrogenation of reaction is fully reversible, in the presence of a suitable catalyst.
C6H6 + 3 H2⇌ C6H12
The hydrogenation reaction is highly exothermic. Provided it is possible to recover this heat, the overall efficiency for storage process could be as high as 98%; without heat recovery, the efficiency is around 77%.
Another option is methanol (CH3OH). This is usually manufactured from syngas by the catalytic reaction of two molecules of hydrogen with one of carbon monoxide. This can either be used as a hydrogen “carrier” or directly as a fuel. It is unlikely that it would be manufactured from hydrogen produced by electrolysis and then decomposed back to hydrogen as the energy efficiency of such a cycle would be very poor. However, methanol derived from fossil fuel is a prime candidate for fuel cells in road transportation and other portable applications.
Hydrogen may also be stored chemically in the form of soluble ionic salts of the form Na+[AHx]–, where A represents boron or aluminium; such salts are generally known as “complex hydrides”. For hydrogen storage, the aluminium salts Na[AlH4] and Na3[AlH6], are preferred. In the case of Na[AlH4], thermal decomposition is in two stages.
3Na[AlH4] ⇌ Na3[AlH6] + 2Al + 3H2
Na3[AlH6] ⇌ 3NaH + Al + 3/2H2
The above reactions are only reversible at elevated temperatures and pressures. The first reaction occurs at 50-100°C and results in the release of 3.7 wt.% hydrogen. The second reaction occurs at 130-180°C and releases a further 1.9 wt.% hydrogen. Research indicates that, in the presence of a titanium catalyst, the temperatures for discharge and recharge of hydrogen may be reduced. It is interesting to note that, even if only the first reaction occurs, the hydrogen storage in terms of wt.% using Na[AlH4] is higher than that offered by AB, AB2, or AB5 metal hydrides.
Sodium borohydride (NaBH4) remains stable until about 400°C. It is therefore unsuitable for providing hydrogen through a thermal activation process. It does, however, release hydrogen in contact with water.
NaBH4 + 2H2O → NaBO2 + 4H2
In the reaction, which is irreversible, NaBH4 not only acts as a hydrogen carrier but also as a “water-splitting” agent; 50% of the hydrogen produced in the reaction comes from the water. The amount of hydrogen produced is about 21 wt.%, based on the mass of NaBH4.
A drawback to using chemical hydrides is that the spent solution has to be returned to a processing plant for regeneration of the hydride.
4. Hydrogen Transportation
At present, hydrogen is transported from where it is produced to where it will be used by land and sea. On land, it is transported via pipeline and over road and rail in cryogenic liquid tanks or compressed gas cylinders. By sea, it is transported in cryogenic liquid tanks or using hydrogen “carriers”. At its point of use, additional infrastructure components may be required. Compression, storage, dispensers, meters, and contaminant detection and purification technologies are commonly used.
Hydrogen transportation and distribution present specific concerns in terms of safety. These concerns are principally related to its chemical and physical properties. They are common to all methods of transportation and are discussed in relation to the development of codes and standards later in this article.
4.1. Pipelines
Pipelines are used where there is a significant demand for a product, which is expected to remain so for the foreseeable future. In the case of hydrogen gas, there are a lot of similarities with the transportation of natural gas. A lot of work is currently being done to evaluate whether natural gas pipelines can be converted to hydrogen. Where new hydrogen gas pipelines are being planned, existing data on emissions and energy requirements for natural gas pipelines may be used in their design.
Pipelines are also used to transport liquid hydrogen “carriers” such as ammonia, cyclohexane, and methanol; but not in the same pipeline as hydrogen gas, as the design requirements are quite different. Ammonia and methanol have been transported by pipeline for many years, so the technology is well established. In the case of cyclohexane, it should be possible to utilise the existing infrastructure used to transport fossil fuels.
For the reasons discussed in relation to Figure 1, it is not practical to transport liquid hydrogen by pipeline
As noted above, the transportation of hydrogen presents specific concerns in relation to safety. There are a number of additional concerns in the case of pipelines. These are addressed in the relevant codes and standards. ASME B31.12 is an international standard that defines the criteria for the compatibility of new and existing steel piping for the safe transport of hydrogen.
4.2. Road and Rail
In the case of hydrogen gas, before the hydrogen can be transported it needs to be compressed. The energy demand for compression depends primarily on the initial pressure of the hydrogen to be compressed. If it is assumed that hydrogen needs to be compressed from 20 to 200 bar, 0.7 kWh/kg of hydrogen will be required. Because of leakages during production, transportation, and distribution, it can be expected that approximately 4% of the total inventory will be lost. Additionally, a small amount of hydrogen remains within the cylinders after the gas has been discharged; this cannot be used.
In the case of liquid hydrogen, a conservative estimate of energy required for the liquefaction process is 10 kWh/kg hydrogen. It should be noted that this figure also considers the energy demand for auxiliaries, flash gas management, losses, and boil-off during the liquefaction process. Even though modern storage tanks reduce boil-off losses to a minimum, it is still assumed that roughly 1% of the liquefied hydrogen will be lost due to boil-off during storage at the refuelling facility.
The decision as to whether to transport hydrogen as a gas or a liquid comes down to cost and how the hydrogen is stored at the production facility.
The design and materials concerns in relation to the transport of hydrogen by road and rail are essentially the same as those relating to its storage. Where hydrogen is be transported by road or rail using a hydrogen “carrier”, again the technologies are well established so there should be no additional concerns with this method of transportation.
4.3. Sea Transport
It is not cost effective to transport hydrogen by sea other than as a liquid. The first liquid hydrogen tanker, the “Suiso Frontier”, has recently been launched. The liquid hydrogen is transported at atmospheric pressure at temperature of minus 253°C; at pressure and temperature, it occupies approximately 0.1% of the volume it originally occupied as a gas. The vessel in question has one, 1250m³ vacuum-insulated storage tank and will transport liquid hydrogen from Australia to Japan.
Whilst many of these challenges involved in transporting liquid hydrogen at sea are similar to those experienced when transporting liquid natural gas (LNG) at minus 160°C, the transportation of liquid hydrogen presents some unique challenges. For example, onboard LNG tankers, nitrogen is used to provide an inert atmosphere around the storage tank(s) and associated equipment. This is not possible on a liquid hydrogen tanker as the nitrogen becomes liquid at minus 196°C and would condense if it came into contact with a surface at minus 253°C. Without an inert atmosphere, oxygen presents a similar challenge. It has a boiling point of minus 192°C so, if air was to come into contact with a surface at minus 253°C, liquid oxygen would form, which would be extremely hazardous. Helium could be used to provide an inert atmosphere, but it is extremely expensive and maintaining an adequate gas shield would be exceptionally difficult. For this reason, using a vacuum is the only real option.
In order to manage the problem of boil off onboard, the liquid hydrogen storage tanks need to be designed to provide a degree of pressure containment. However, because of the very limited envelope within which liquid hydrogen exists (see Figure 1), the Suiso Frontier has also been fitted with a Gas Combustion Unit (GCU) to deal with excess boil-off. The problem of boil-off is not unique to liquid hydrogen tankers, similar issues exist with LNG tankers. One option for future, is to use the boil-off gas to power the main engine.
Other than certain specific issues, the design/materials concerns in relation to the transport of liquid hydrogen at sea are essentially the same as those relating to its storage on land.
5. Code Considerations
As already discussed, hydrogen production, storage, and transportation present specific issues in terms of safety. These issues relate primarily to the chemical and physical properties of hydrogen: its ability to embrittle materials, its ease in escaping from containment, its wide flammability range, and the limited amount of energy needed to ignite it, which all represent obstacles to its safe use.
If the hydrogen economy is to succeed, any technological developments have to be made in parallel with the development of codes and standards specific to hydrogen. These will not only assure its safe use but will allow manufacturers to play in a regulated field that couples the protection of the customer with competition in the marketplace.
At an international level, ISO Technical Committee 197 has been charged with developing standards related to hydrogen applications. Some standards are available for specific applications, such as piping and pipelines (ASME B31.12), but others are still under development. As a result, local regulations are being implemented. These are generally based on natural gas regulations with ad hoc countermeasures introduced to cover the peculiarities of hydrogen gas.
In adapting natural gas regulations on distribution networks, it is important to understand the safe distances that need to be imposed between hydrogen installations and the nearest buildings. The need for an accurate evaluation of these distances is particularly relevant in the case of hydrogen because the industry is in its infancy and tough restrictions may deter its wider public acceptance.
6. References
1. Leung, W. B., et al., Phys. Lett. A (1976) 56, 425