I do not wish to go into too much detail in this article, but before discussing the effect of the cooling rate, I need to mention the effect of the heating rate on the A1 and A3 lines. They are not fixed. They vary with the heating rate and because the heating rates used in industrial applications are generally higher than those used in controlled laboratory experiments, the A1 and A3 temperatures tend to be a few degrees above the transformation temperature shown in Figure 1.
The A1 and A3 temperatures that occur on heating are known as the austenite start temperature, Ac1, and the austenite finish temperature, Ac3. It is the Ac1 and Ac3 temperatures that are generally referred to rather than the A1 and A3 temperatures when discussing heat treatment cycles.

Heat Treatment Cycles – With regard to heat treatment cycles, carbon steels are generally supplied in one of three heat-treated conditions:
1) Annealed Condition – When it is specified that a component is to be supplied in the annealed condition, this normally implies that the steel is to be fully annealed. Full annealing is generally carried out to remove the effects of cold-working and involves heating the steel to a temperature approximately 15-30°C above the Ac3 line (Figure 2) and holding it there for sufficient time to allow it to transform to a fully austenitic grain structure The annealing process may be divided into three stages (Figure 3):
- Recovery – This occurs at temperatures below the Ac1 line. It does not alter the microstructure and any changes in the mechanical properties are small. The principal effect of the recovery stage is to relieve the residual stresses built up in the material due to cold working. (Note: commercially, this low temperature treatment, where the material is kept within the recovery range, is known as stress-relief annealing or process annealing. As the temperature does not exceed the Ac1 line, there is no phase transformation so the cooling rate is immaterial).
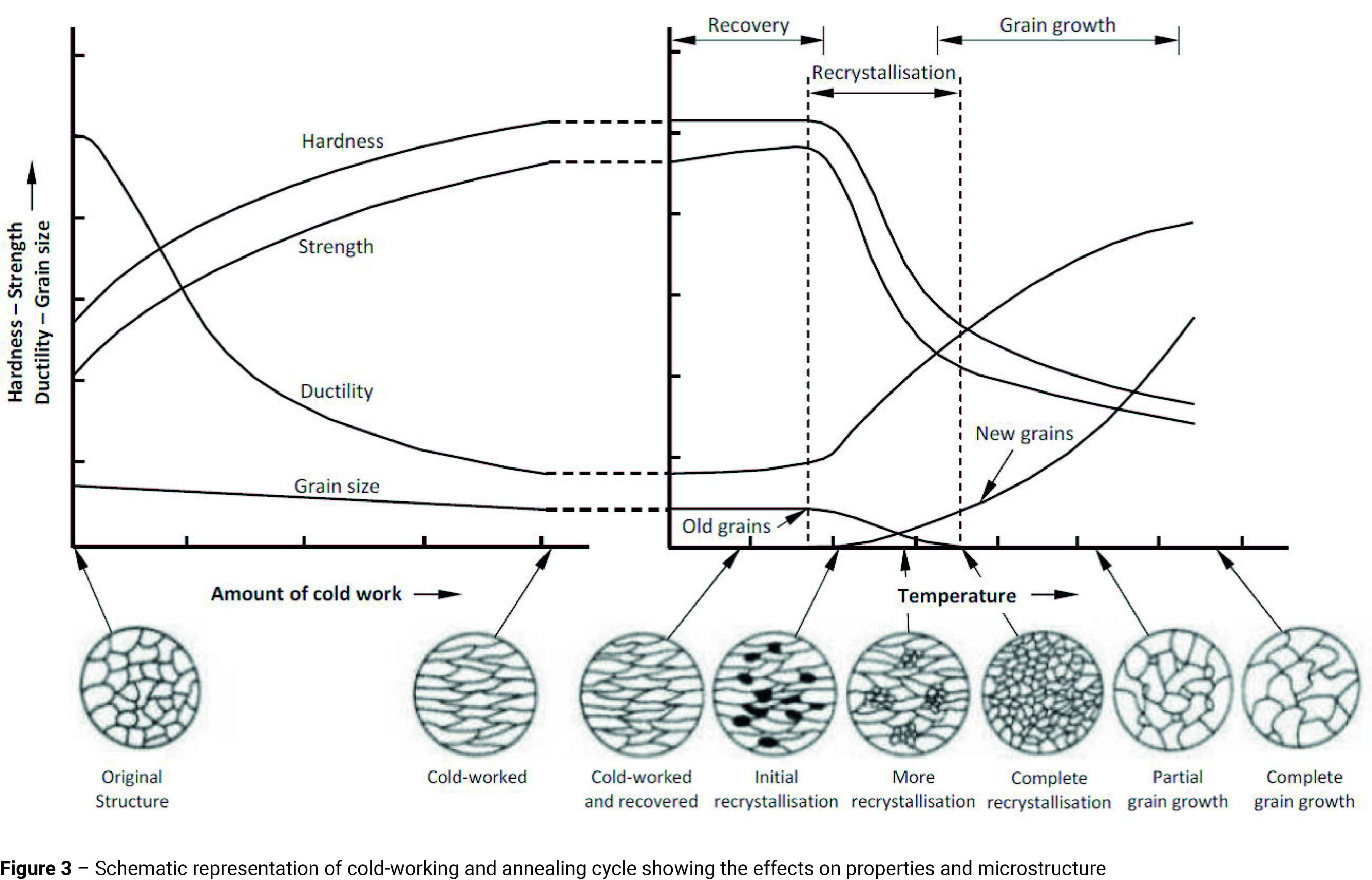
- Recrystallisation – As the temperature reaches the upper limit of the recovery zone, minute new crystals start to appear in the microstructure. These new crystals have the same composition and lattice structure as the existing grains, but are equi-axed, i.e. they are not elongated. The new crystals generally form at the most severely deformed parts of the grain, usually at the grain boundaries and slip planes. The clusters of atoms from which the new grains are formed are called nuclei. Recrystallisation occurs through a combination of nucleation of strain free grains and the growth of these nuclei until the entire cold-worked material has been absorbed.
The term recrystallisation temperature does not refer to a specific temperature below which recrystallisation will not occur, rather to the approximate temperature at which a highly cold-worked material completely recrystallises in one hour. The temperature at which recrystallisation begins is dependent upon the degree of prior deformation; the greater the prior deformation, the lower the temperature at which recrystallisation will begin. During the recrystallisation stage, there is a significant drop in the tensile strength and hardness, and a large increase in the ductility of the material (Figure 3).
- Grain growth – Heating the steel to just above the Ac3 line initially creates austenitic grains which are smaller than the previous ferritic grains, but as the material reaches the final heat treatment temperature and is held there grain growth occurs and eventually the grains return to their original size (Figure 3). During the grain growth stage the tensile strength and hardness continue to decrease, but at a much slower rate than during the recrystallisation stage.
Having been held at the heat treatment temperature for the allotted time, the steel is cooled very slowly so that the equilibrium microstructure is obtained. To achieve the required cooling rate, the steel would normally be kept in the furnace. The furnace would be turned off and the steel allowed to cool down inside. The fundamental requirement is that the cooling rate is slow enough to ensure that the austenite transforms to ferrite and pearlite.
In the fully annealed condition, the microstructure resembles that predicted by the stable iron-carbon phase diagram (Figure 1). An example of the microstructure of an annealed carbon steel is shown in Figure 6. As noted above, annealing is used primarily to remove the hardening effects that occur as a result of cold-working. The steel will exhibit relatively low levels of hardness, yield strength and ultimate tensile strength, but a high level of ductility.
2) Normalised Condition – The normalising process is similar to the annealing process in that it also involves heating the steel to a temperature 20-50°C above the Ac3 line (Figure 2) and holding it there for sufficient time to allow it to transform to a fully austenitic grain structure. However, that is where the similarity ends. The period during which the steel is held above the Ac3 line is called the soak time. At the end of the soak time, the steel is removed from the furnace and allowed to cool in air. Because the cooling rate is much faster, recrystallisation occurs and new ferritic grains form. The resulting microstructure is far more refined (i.e. smaller grain size) than it was prior to normalising. The steel is stronger and tougher than it was prior to normalising or than it would be in the fully annealed condition (Figures 4 and 5).
Normalising is usually carried out to obliterate the effects of forming operations (i.e. hot and cold working) and to achieve a defined level of strength and toughness. It is also carried out to ensure a homogeneous austenitic grain structure on reheating for quenching or full annealing. Depending on the composition of the steel, the resultant microstructure will be pearlite or pearlite with excess ferrite or cementite. The microstructure differs from that produced by annealing; for steels of the same carbon content, there will be less excess ferrite or cementite and the pearlite will be finer. These are the results of the more rapid cooling rate. An example of the microstructure of a normalised carbon steel is shown in Figure 7. Since the microstructure is affected by the cooling rate, the mechanical properties in normalised steels can vary considerably when there are differences in the section thicknesses of the shapes being normalised.

3) Quenched and tempered – Quenching and tempering is performed in two stages. The steel is first hardened to produce a microstructure providing high strength, but low toughness. The steel is then tempered to obtain the desired balance of strength and toughness.
- Quenching – Steels can be hardened by being heated to a temperature approximately 15-30°C above the Ac3 line (Figure 2), being held there long enough to ensure that the temperature of the steel is uniform and the structure is fully austenitic (in a similar manner to annealing), then cooled rapidly (quenched). Steels behave differently depending on the severity of the quench (cooling rate). A useful tool for predicting the behaviour of steels is the time-temperature-transformation (TTT) diagram.
Figure 8 shows a typical TTT diagram. The TTT-diagram is useful because it presents an overall picture of the transformation behaviour of austenite. It enables the metallurgist to interpret the response of a steel with a given chemical composition to a specified heat-treatment, i.e. annealing, normalising or quenching and tempering. It is particularly useful in determining whether the cooling rate required to obtain the desired microstructure can be achieved. I have included Figure 8 in this article simply to help demonstrate the importance of the cooling rate during quenching. The methods of producing TTT-diagrams and the factors governing their shape are quite complex and will be discussed in detail in a later article.
As I explained in my article “Fundamentals of Carbon Steel Part 1”, if cooling is sufficiently rapid, the carbon atoms are unable to diffuse through the lattice, such that austenite is unable to transform to ferrite/pearlite. With reference to Figure 8, if the temperature can be reduced to below the MS line before the austenite is able to transform, martensite will begin to form (i.e. the temperature is reduced rapidly enough such that the cooling curve misses the nose of the S-curve).
Provided the cooling rate is sufficiently rapid and the final temperature is below the MF line, the resulting microstructure will be fully martensitic. (Note: MS is the martensitic start temperature; that is the temperature at which martensite starts to form. MF is the martensitic finish temperature and is the temperature below which martensite will no longer form. The MF line is really only a concern when it is below ambient temperature; where this is the case, significant amounts of untransformed, or retained austenite, may be present, intermingled with martensite. Because retained austenite exists outside of its normal temperature range, it is metastable. This means that given the opportunity, it will transform to martensite. This transformation is accompanied by a volume change that induces high internal stresses, which can often result in cracks.).
I have explained the significance of the MS and MF lines in the TTT-diagram, but there is another line that is equally important in the quenching and tempering operation; that is the BS line. The BS line indicates the temperature at which bainite begins to form. As I discussed in my article “Fundamentals of Carbon Steel Part 1”, with normal carbon steels, where high strength and good toughness are required the optimum microstructure is bainitic (Figure 9).
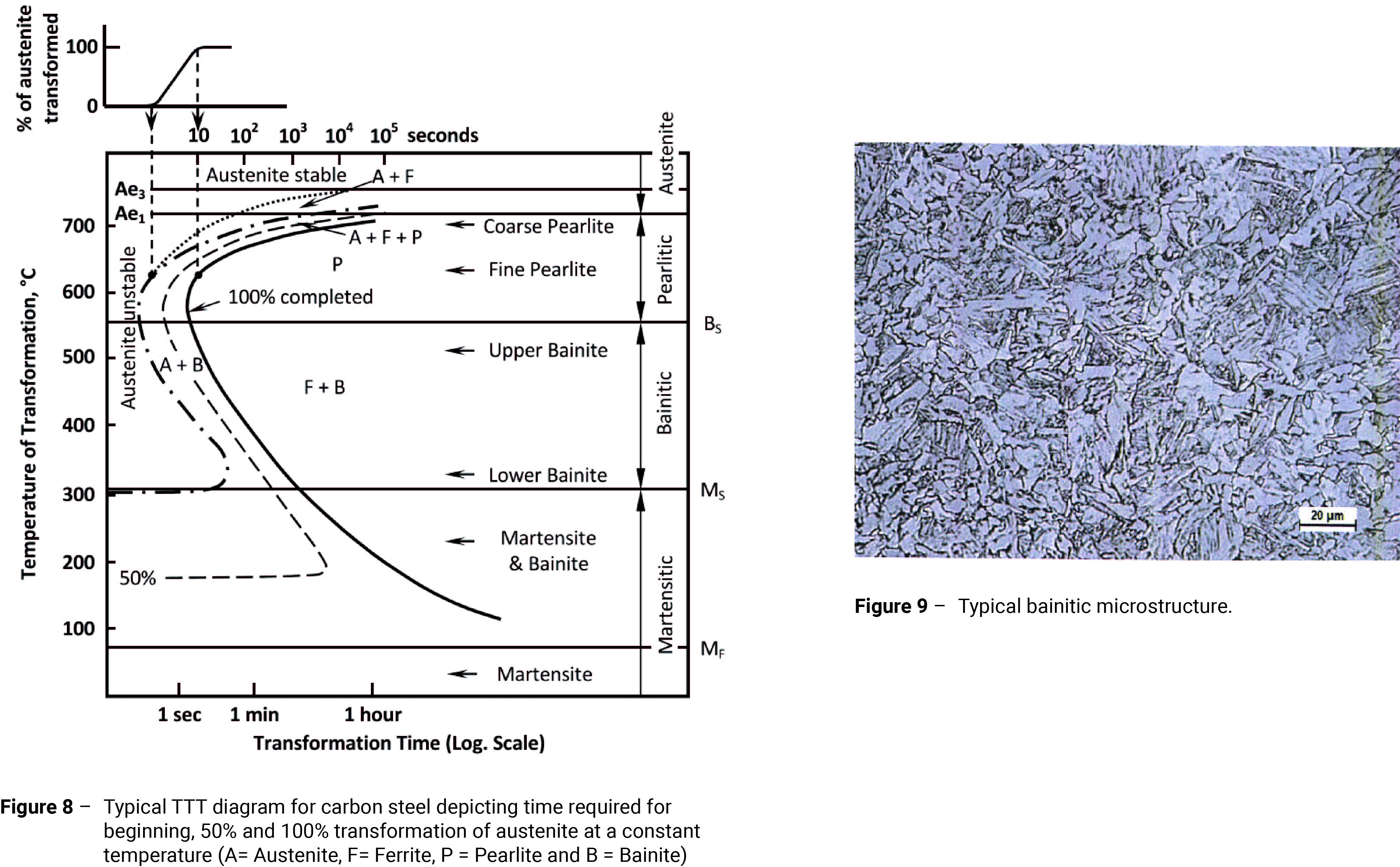
As both martensite and bainite are inherently brittle, steel is rarely used in the as-quenched condition. It is given a further ‘tempering’ heat treatment.
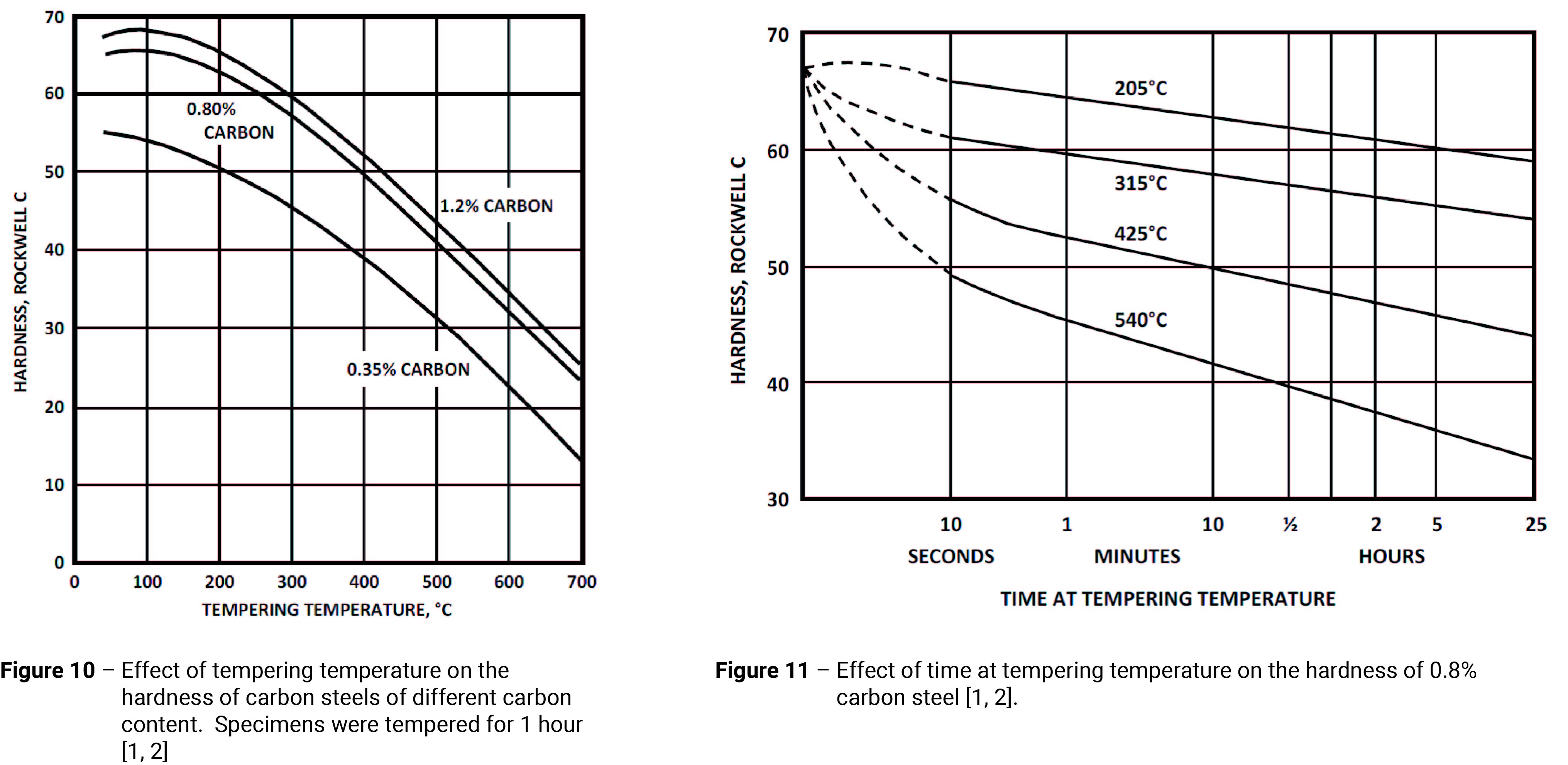
- Tempering – Tempering is a heat treatment technique applied to hardened steels to achieve greater toughness by reducing its strength/hardness. Tempering involves heating the steel to a temperature below the Ac1 line (Figure 2) and holding it there for a predetermined time. The tempering precise temperature and time depend on the desired properties and the purpose for which the steel is to be used. If high strength/hardness is required, the tempering temperature needs to be low; if good toughness is required, the tempering temperature needs to be high.
Figure 10 shows the effect of the tempering temperature on the resultant hardness of fully hardened carbon steels. However, for any given temperature the tempering time is also important. As Figure 11 demonstrates, the hardness drops off rapidly once the tempering temperature is reached, then continues to decrease more slowly with time. Whilst Figure 11 would suggest that the steel only needs to be kept at the tempering temperature for a few minutes, short tempering times are generally undesirable and should be avoided. Good practice requires at least ½ hour (or, preferably, 1 to 2 hour) at tempering temperature [1].
The heating and cooling rates used in tempering are largely immaterial. However, an excessive heating rate should be avoided as it can produce steep thermal gradients within the material, which can cause distortion or in severe cases cracking. The cooling rate is irrelevant unless the steel is susceptible to temper brittleness; in which case it should be quenched from the tempering temperature.
In this article, I have described the three basic heat treatment processes applied to basic carbon steels to control their microstructure/mechanical properties. I have discussed how, depending on the cooling rate, different microstructures occur, i.e. ferrite/pearlite, bainite and martensite. In my next article, I will explain how these microstructures form as a result of decomposition of austenite.
References
1. THOMAS G. DIGGES & SAMUEL J. ROSENBERG, Heat Treatment and Properties of Iron and Steel, National Bureau of Standards Monograph 18, Issued October 3, 1960
2. EDGAR C. BAIN, Functions of the alloying elements in steel, Am. Soc. Metals, Novelty, Ohio (1939) 312 pages