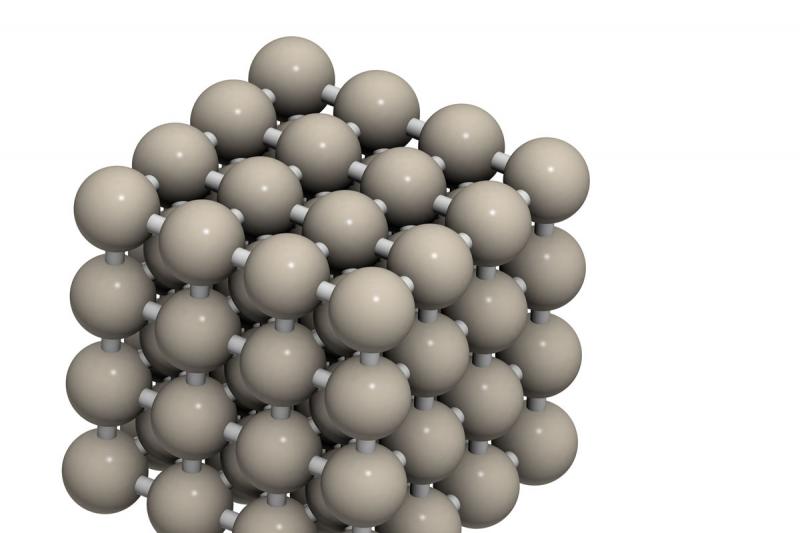
Carbon steel is fundamentally an alloy of iron and carbon. At room temperature, carbon is virtually insoluble in iron; the maximum solubility of carbon is approximately 0.008 wt%. Below 0.008 wt%, the structure will be made up entirely of ferrite, which has a body-centred cubic lattice, i.e. the lattice is in the form of a cube with one atom of iron at each corner and one at the centre (Figure 1). Above 0.008 wt%, any ‘excess’ carbon combines with iron to form cementite, Fe3C. Thus, at room temperature, carbon steels consist of a mixture of two phases, cementite and ferrite.
With reference to Figure 3, at temperatures below the A1 line, hypo-eutectoid steels are comprised of ferrite and pearlite (Figure 4); pearlite is a lamellar structure consisting of platelets of cementite interspersed through the ferrite. When the steel is heated to a temperature above the A1 line, the cementite dissolves to form a new phase austenite. This has a face-centred cubic lattice, i.e. again, the lattice is in the form of a cube with one atom of iron at each corner, but in this case it has one atom of iron at the centre of each face (Figure 2).

The solubility of carbon in austenite is approximately 0.83 wt% (the location of the eutectoid in the iron-carbon phase diagram). Until the A3 temperature is reached, the structure will be a mixture of ferrite and austenite. If the steel is heated to above the A3 temperature any ferrite will transform to austenite and the structure will be fully austenitic.
It is important to note that phases in steel should not be confused with structures. Whilst there are many structures or mixtures of structures, there are only three phases involved in any steel; ferrite, cementite and austenite.
The area denoted as austenite in Figure 3 is an area within which iron can retain much dissolved carbon. The annealing, normalising and ‘quenching’ heat treatment operations begin by heating the steel to above the A3 temperature in order to dissolve the carbide in the iron and produce a fully austenitic structure.
If the steel is cooled slowly from a temperature above the A3 line, transformation to the body-centred cubic ferrite phase begins when the temperature drops below the A3 line. As the temperature continues to decrease, the transformation is essentially complete once the A1 line is reached. During this transformation, the carbon atoms are expelled from the lattice because they are essentially insoluble in the body-centred cubic ferrite. To all intents and purposes, the steel is returned to the same state it was in before it was heated to form austenite.
Under normal circumstances, austenite cannot exist at room temperature in carbon steel. However, the rate at which carbon steel is cooled from the austenitic range has a profound influence on its room temperature microstructure and mechanical properties.
When the steel is cooled rapidly, the carbon atoms are unable to diffuse through the lattice and become trapped causing distortion of the lattice. This distortion manifests itself in the form of hardness and/or strength. If cooling is rapid enough, a new structure called martensite will be formed.
Martensite is not a phase. It is a specific microstructure in the ferritic phase. Martensite is formed when steel is cooled rapidly from above the A3 temperature such that the carbon atoms do not have time to diffuse through the lattice to form cementite and effectively lock the lattice of the austenitic atomic arrangement in a distorted body-centred tetragonal structure, i.e. the lattice is in the form of a tetrahedron with one atom of iron at each corner and one at the centre (Figure 5). Thus, martensite should be considered as an aggregate of ferrite and cementite. A typical martensitic microstructure is shown in Figure 6.
At slightly slower cooling rates, another structure known as bainite may form (Figure 7). This ability to change the structure of carbon steel by heat treatment is extremely useful and allows the engineer to select the right balance of strength, toughness and hardness (wear resistance) depending on the application. For most process piping applications, because of the way the allowable stress is established, the optimum microstructure is one of ferrite/pearlite; this has a relatively low yield strength to tensile strength ratio, which means the material is naturally ductile. In order to ensure it has adequate toughness at low temperatures, it is important it has a fine-grain structure (Figure 8) and for this reason the material will normally be ordered in the normalised heat treated condition, i.e. the steel is heated to above the A3 temperature and cooled in air.
For pipeline applications, where the allowable stress is dictated by the yield stress and the yield strength to tensile strength ratio is not so important; for normal carbon steel forgings, the optimum micro-structure is bainitic (Figure 7). This offers an excellent combination of strength and toughness, and is achieved by heating the steel to above the A3 temperature and cooling it rapidly in water, i.e. quenching. It is likely that during this operation a thin layer of martensite will be produced at the surface, but this will not extend very far into the steel and is likely to be removed during subsequent machining of the forging.

It can be seen that heat treatment and principally the cooling rate is critical to obtaining the required microstructure in carbon steel. The simplest approach explaining the effect of cooling rate on restructure is to consider a time-temperature-transformation (TTT) curve, but this is a subject for another blog.
References
1. W. Callister, Materials Science and Engineering - An Introduction, Utah, John Wiley & Sons Inc, 2000
2. Martensite Photomicrograph Copyright © British Steel; Used with permission, Courtesy of Corus.