1. INTRODUCTION
This is Part 3 of a series of articles covering the invention and development of Alloy 625. In Part 2, I covered the solidification behaviour of Alloy 625 and how this affects the melting practices. I discussed the metallurgy of Alloy 625 in relation to how even small variations in the chemical composition can have a big impact on the properties of the finished product. In this article, again based on a paper produced by Stephen Floreen, Gerhard E. Fuchs, and Walter J. Yang [1], I cover the metallurgy of the solid state alloy; I discuss the precipitation of γ”, the various carbides, and Laves and Delta phase. I also consider how tighter control of alloy content and processing steps can be used to optimise different properties.
2. PHASE TRANSFORMATIONS
For the purposes of this article, it is assumed that the starting material has been solution annealed such that, with the exception of the primary niobium carbide (NbC) particles discussed in Part 2, all other phases have been put into solid solution.
The starting point for explaining the phase transformations that can occur in Alloy 625 is the time-temperature-transformation (T-T-T) diagram. A number of time-temperature-transformation (T-T-T) diagrams have been proposed for Alloy 625; all of which differ slightly from each other. In this article, the T-T-T diagram shown in Figure 1 is used. This represents the T-T-T diagram for nominal heats of wrought Alloy 625, with compositions within the range shown in Table 1.
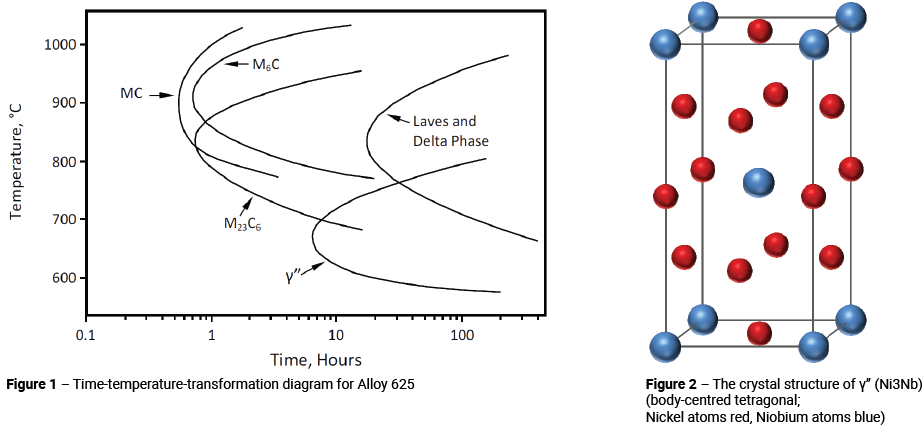
The main thing to note in Figure 1 is the γ” curve. If sufficient niobium, titanium and aluminium are present, γ” precipitates as a fine Ni3(Nb + Ti + Al) dispersoid in the temperature range 590 to 760°C. The crystal structure of γ” is body-centred tetragonal (BCT) with an ordered arrangement of nickel and niobium atoms, as shown in Figure 2. γ” precipitates are disc-like in shape and form as a result of the lattice mismatch between the BCT γ” and the face-centred-cubic (FCC) Alloy 625 matrix. This lattice mismatch leads to high coherency strains which, together with order hardening, comprise the primary strengthening mechanisms.
In addition to γ”, it can be seen that a number of different carbides and intermetallic phases can also precipitate in Alloy 625 after thermal exposures, for times of the order of 0.1 to 100 hours.

2.1. γ” Precipitation
Alloy 625 was not originally developed as a precipitation hardened alloy. As noted above, γ” precipitation is dependent on the levels of niobium, titanium and aluminium, and small changes in composition can have a marked affect on the propensity of γ” to precipitate. Figure 3 shows the influence of the titanium content. It is based on the results from a series of samples taken from melts that all contained approximately 3.85% Nb, but were otherwise nominally constant in composition [1]. Figure 3 indicates that reducing the titanium content from 0.4% to 0.05% and then to zero significantly retards the precipitation of γ”. Figure 4 shows the influence of the aluminium content indicating that reducing the aluminium content to zero has much less effect than in the case of titanium. Microstructural examinations carried out on the various samples showed that only γ” was involved in precipitation hardening in these tests [1]. No γ’ or other phases that could contribute to hardening were detected. Similar effects of chemistry have been observed by Garzarolli et al [2]. Consistent with the results shown in Figures 3 and 4, they postulated that the individual effects of niobium, titanium and aluminium could be explained in terms of precipitation of an Ni3(Nb + Ti + Al) γ” precipitate.

It is not only changes in composition that affect γ” precipitation, variations in processing history can also influence the precipitate morphology. If NbC, Laves or Delta particles are present around the grain boundaries, the area adjacent to these particles will be depleted of niobium. Where this is the case, the formation of γ” may be retarded resulting in zones denuded of γ”. In other precipitation hardened alloys, the presence of such zones has often been associated with degradation in mechanical properties and/or corrosion resistance. However, there is very little information about the specific effects of such zones in Alloy 625. Although it is relatively straightforward to explain why local denudation in γ” may be observed in Alloy 625, there is not yet sufficient information to define how the prior processing history affects the γ” precipitation behaviour and resultant properties in Alloy 625.
2.2. Carbides
As indicated in Figure 1, there are three different carbides that can form in wrought Alloy 625 and will be present as grain boundary precipitates. The positions of the noses of the various carbide curves are not well defined, but occur at around 10 minutes. This suggests that even in fairly large Alloy 625 components grain boundary carbides will not form after air cooling from solution annealing. Although in practice, most manufacturers would tend to use some form of accelerated cooling, e.g. water quenching.
The type of carbide that forms is dependent on the temperature. At higher temperatures, in the range 870 to 1038°C, the carbides that form are both NbC (as thin grain boundary films), and MC, where M is principally Ni, Cr, and Mo. At temperatures in the range 705 to 915°C, the carbides are primarily M23C6, where M is almost entirely Cr. After intermediate temperature heat treatments in the region of 870°C, all three carbides usually can be found. The M6C and M23C6 carbides generally have blocky irregular shapes and form as series of separate, discrete grain boundary particles [1].
It has been found that the precipitation of grain boundary carbides is sensitive to the silicon content as well as the carbon content. At carbon levels above about 0.035%, silicon appears to have no effect, but at lower carbon levels, carbide precipitation appears to be significantly retarded when the silicon contents are below about 0.15%. The reason for this strong effect of silicon is not clear, but it has been noted that M6C carbides in Alloy 625 contain about 5 wt % silicon, which suggests that silicon might promote the formation of M6C [1, 3]. The influence of the silicon content serves as a good illustration of how a minor change in chemistry can have a significant effect on the metallurgical behaviour of the alloy.
Grain boundary carbides are detrimental not only to the corrosion performance of the alloy, but also its mechanical performance. The presence of M6C and M23C6 precipitates in particular can drastically reduce the ductility and toughness. Fortunately, carbides can be easily eliminated by solution annealing at 1093°C or higher. At 1093°C, they will usually be eliminated after an exposure time of about one hour.
2.3. Intermetallics
With reference to Figure 1, it can be seen that with longer exposure times, both Laves and Delta phase will start to precipitate. Precipitation begins in regions of the grain boundaries that already contain carbides.
The structure of Laves phase was discussed in Part 2. Laves particles have a morphology very similar to that of the blocky irregular shaped M6C and M23C6 carbides. Delta phase has an orthorhombic D0a structure, as shown in Figure 5. Delta phase particles have an acicular morphology. Laves and Delta phase precipitation are shown as a single curve in Figure 1 and although this may not be accurate in all cases, because both phases depend upon the diffusion of niobium for their formation, the kinetics of the precipitation reaction should be very similar in each case.
At temperatures towards the bottom of the curve, Delta phase nucleates at grain boundaries as well as coherent and incoherent twin boundaries. At higher temperatures, it precipitates intragranularly [4].
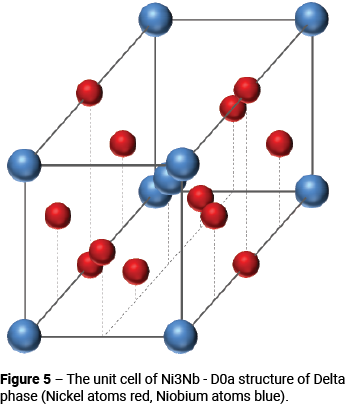
Delta phase can also form as a transformation product of γ”, which becomes unstable above about 650°C [5, 6, 7]. Although Delta phase precipitates are incoherent and their precipitation is known to reduce the strength of γ” strengthened alloys, controlled precipitation of Delta phase has some beneficial effects like grain stabilisation [8].
Whilst their effects are not as severe as those of M6C and M23C6 carbides, the presence of Laves and/or Delta phase will reduce the ductility and toughness. Laves and Delta phase can both be put back into solution by annealing at 1093°C; however, much longer annealing times are required than in the case of carbides.
3. COMPOSITIONAL FINE TUNING
The structures and properties of individual heats of Alloy 625 can vary considerably depending upon the processing history and chemical composition of each heat. For critical applications, in order to ensure consistency in the properties on a heat-by-heat basis, more restrictive compositional limits than those given in Table 1 may be required. To achieve this successfully requires a consideration of the trade-off in properties that may result when such compositional modifications are made.
A starting point is to consider how compositional changes affect the microstructure. Table 2 summarises some of the compositional effects in relation to the formation of different phases during solidification and heat treatment.

Laves and NbC particles formed during solidification, and Laves and Delta phase particles formed during heat treatment are detrimental phases that should be avoided. Although grain boundary carbides are generally considered to be deleterious to the performance of the material, certain types and distributions may be beneficial for stress corrosion resistance (in certain environments). In addition, if high strengths are required, γ” precipitates are needed. Therefore, the initial step is to determine whether grain boundary carbides and/or γ” precipitation hardening are required.
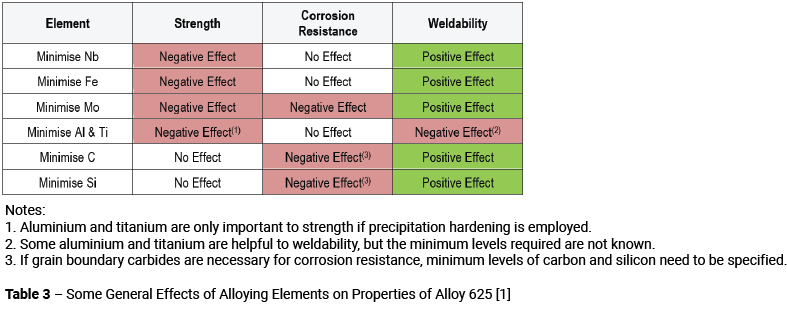
The next step is to consider how the various alloying elements affect the principal material properties. Table 3 presents an overview of how changing the composition might affect the mechanical properties, corrosion resistance and weldability of the material. It should be recognised that for specific applications, it is necessary to accurately evaluate the effects of changes in the composition on the properties of concern and in this respect Table 3 may be inappropriate. The principal point to be made is that fine tuning the composition requires a consideration of all the consequences of the composition change to ensure that solving one problem does not create a new one.
A final consideration is cost. Overly restrictive chemistry ranges can be expensive, and should not be imposed if not necessary.
REFERENCES
- Stephen Floreen, Gerhard E. Fuchs, and Walter J. Yang, “The Metallurgy of Alloy 625”, Superalloys 718, 625, 706 and Various Derivatives, The Minerals, Metals and Materials Society, 1994.
- F. Garzarolli, A. Gersha, and K. P. Francke, Zeit. Metalk. Vol. 60, 1969, p. 643.
- R. Cozar, M. Rouby, M. Mayonobe and C. Morizot “Superalloy 718/625”, TMS 1991, p. 423.
- I. Kirman, “Precipitation in the Fe-Ni-Cr-Nb system,” J. Iron and Steel Inst., 207 (1969), 1612-18.
- M. Sundararaman, P. Mukhopadhyay, and S. Banerjee, “Precipitation of the δ-Ni3Nb phase in two nickel base superalloys,” Metall. Trans. A, 19 (1988), 453-465.
- E. Andrieu, N. Ang, R. Molinis, and A. Pineau, “Influence of compositional modifications on thermal stability of alloy 718,” Superalloys 718, 625 and Various Derivatives, ed. E.A. Loria, (TMS, 1994), 695-710.
- O.B. Armida, and J.F. Radavich, “A current T-T-T diagram for wrought alloy 718,” Superalloy 718, 625 and Various Derivatives, ed. E.A. Loria, (TMS, 1991), 325-335.
- Sundararaman Mahadevan, Sachin Nalawade, Jung Bahadur Singh, Amit Verma, Bhaskar Paul and Kishore Ramaswamy, “Evolution of δ Phase Microstructure in Alloy 718”, 7th International Symposium on Superalloy 718 and Derivatives, TMS 2001, p. 737.